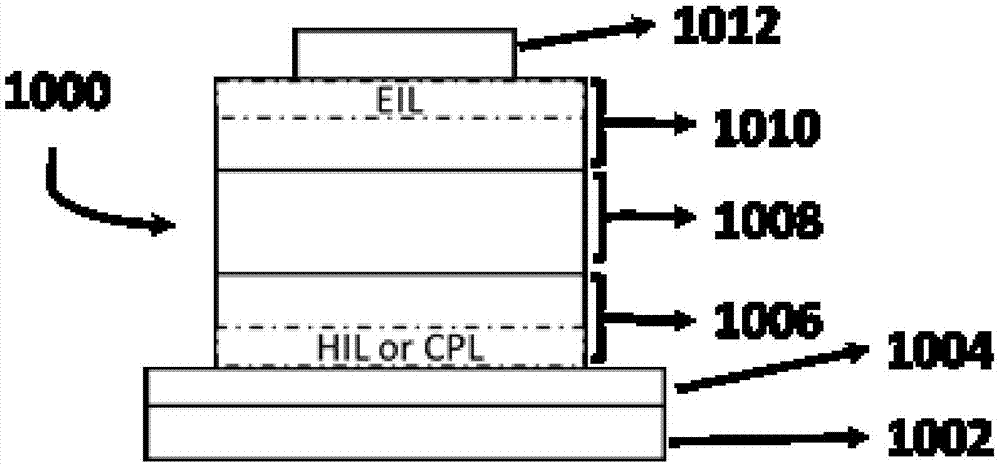
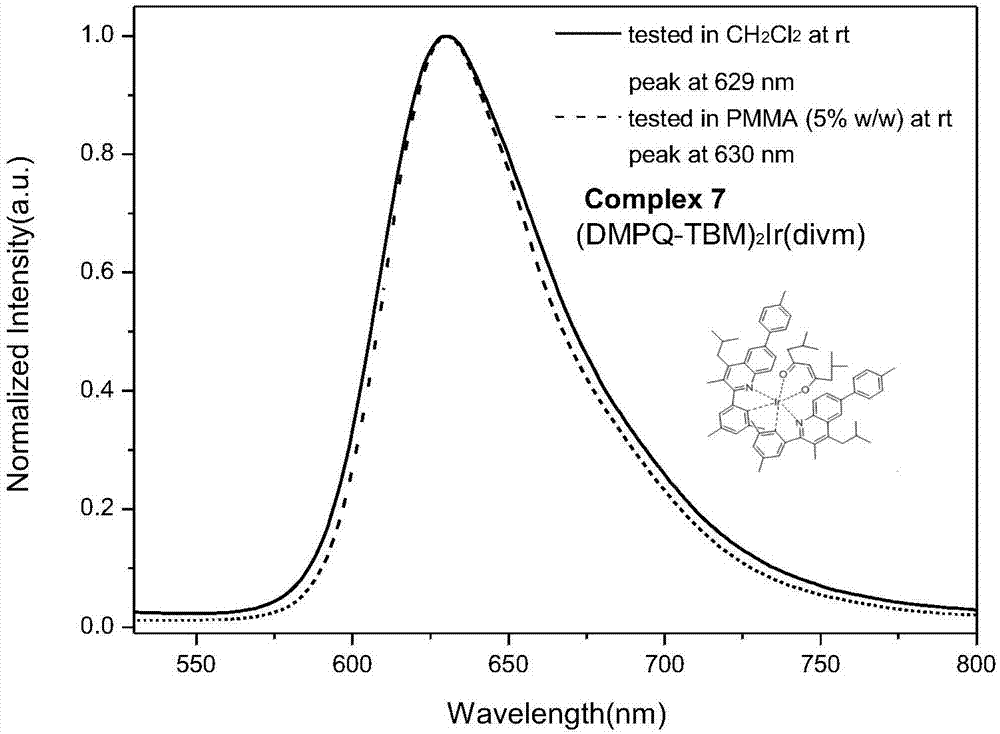
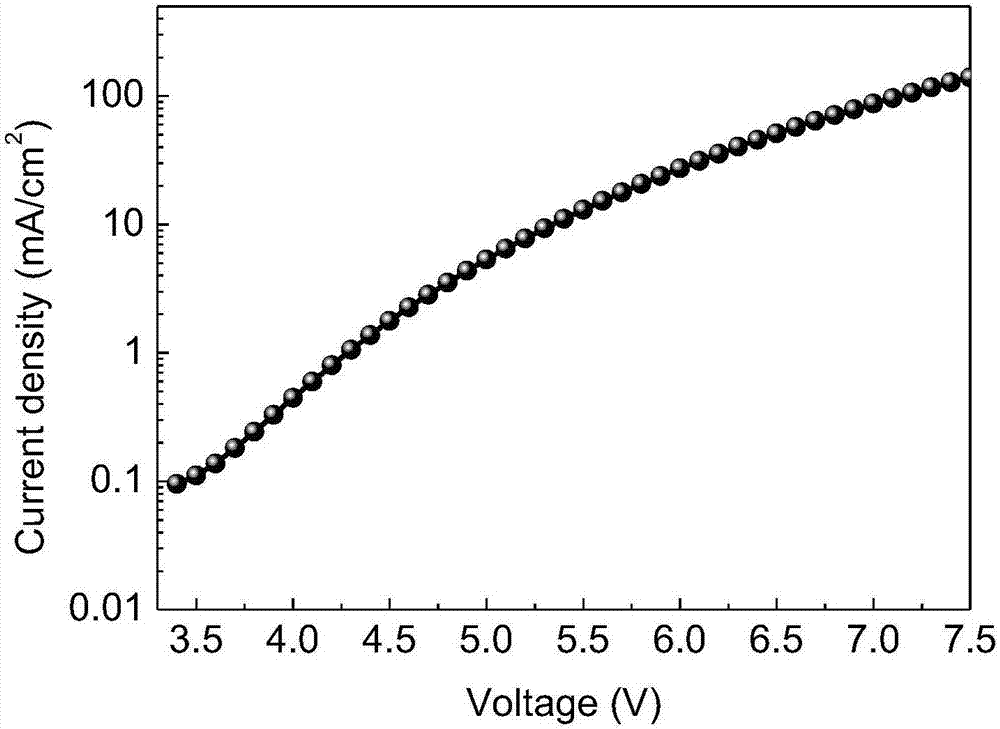
Polysubstituted quinoline-coordinated iridium-hybridized compound as well as preparation method and application of compound
A compound and multi-substitution technology, applied in indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problems of high cost, high material labor and material cost, and difficult purification, and achieve high-efficiency mass production. cost, and the effect of improving fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of embodiment 1 iridium complex 11
[0065] R a = p-tolyl, R b = methyl, R c = phenyl, R d = methyl, R e = Hydrogen, R f = Methyl
[0066](1) Synthesis of N-(4'-methyl-[1,1'-biphenyl]-4-yl)-3-oxo-2-phenylbutanamide. Add 4'-methyl-[1,1'-biphenyl]-4-amine (1g, 5.5mmol), ethyl 2-phenylacetoacetate (3.1g, 15mmol) and xylene ( 15 mL), reaction system N 2 After bubbling for three minutes, the temperature was slowly raised to 145°C, and stirred at 145°C for 16 hours. TLC (petroleum ether: ethyl acetate = 5:1) showed that the reaction was complete, the reaction solution was cooled to room temperature, the product was precipitated, filtered, and dried in air to obtain pale yellow N-(4'-methyl-[1,1'- Biphenyl]-4-yl)-3-oxo-2-phenylbutanamide (500 mg, 26% yield). 1 H NMR (300MHz, DMSO) δ10.40(s, 1H), 7.66(d, J=8.7Hz, 2H), 7.60(d, J=8.7Hz, 2H), 7.53(d, J=8.1 Hz, 2H ), 7.38–7.31 (m, 5H), 7.24 (d, J=7.8Hz, 2H), 5.00 (s, 1H), 2.33 (s, 3H), 2.19 (s, 3H).
[0...
Embodiment 2
[0077] The preparation of embodiment 2 iridium complex 126
[0078] R a = methyl, R b = p-tolyl, R c = Hydrogen, R d = methyl, R e = Hydrogen, R f = Methyl
[0079] (1) Synthesis of 3-oxo-N,3-di-p-tolylpropionamide. Add p-toluidine (2.7g, 25 mmol), 3-oxo-3-p-tolyl-propionic acid ethyl ester (10.3g, 50mmol) and xylene (20mL) to 100ml sealed tube, reaction system N 2 After bubbling for three minutes, the temperature was slowly raised to 145°C, and stirred at 145°C for 16 hours. TLC (petroleum ether: ethyl acetate = 5:1) showed that the reaction was complete, the reaction solution was cooled to room temperature, the product was precipitated, filtered, and dried in air to obtain white 3-oxo-N, 3-di-p-tolylpropane Amide (3.4 g, 60%). 1 H NMR (300MHz, CDCl 3 )δ9.21(s,1H),7.94(d,J=8.1Hz,2H),7.46(d,J=7.5Hz,2H),7.31(d,J=8.1Hz,2H),7.13(d, J=7.5Hz,2H),4.07(s,1H),4.04(br,1H), 2.44(s,3H),2.32(s,3H).
[0080]
[0081] (2) Synthesis of 6-methyl-4-(p-tolyl)quinolin-2-ol. Add 3-...
Embodiment 3
[0090] The preparation method of embodiment 3 iridium complex 201
[0091] R a = methyl, R b = p-tolyl, R c = Hydrogen, R d = Hydrogen, R e = methyl, R f = Methyl
[0092]
[0093] (1) Synthesis of dimer 201. Add 6-methyl-2,4-bis-p-tolylquinoline (61.6 mg, 0.2 mmol), iridium trichloride trihydrate (14.1 mg, 0.04 mmol), ethylene glycol ether ( 3 mL) and water (1 mL), nitrogen was bubbled for three minutes, and the reaction system was heated to reflux for 12 hours. The reaction solution was cooled to room temperature, solid precipitated, filtered, and dried in air to obtain dimer 201 (25 mg, 76%), which was directly used in the next step.
[0094] (2) Synthesis of iridium complex 201. Add dimer (160.6mg, 0.1mmol), pentane-2,4-dione (40mg, 0.4mmol), sodium carbonate (53mg, 0.5mmol) and ethylene glycol ether (3mL) to a 75ml sealed tube, Nitrogen was bubbled for three minutes, and the reaction system was heated to reflux for 12 hours. The reaction solution was cooled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com