Apolipoprotein A1 detection kit and detection method
A detection kit and apolipoprotein technology are applied in the field of medical immunology in vitro diagnosis, which can solve the problems of inaccurate detection results of the reagents, inability to meet the requirements for use, and the coefficient of variation becomes large, and achieve good stability of the reagents, wide versatility, The effect of accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Apolipoprotein A1 detection kit
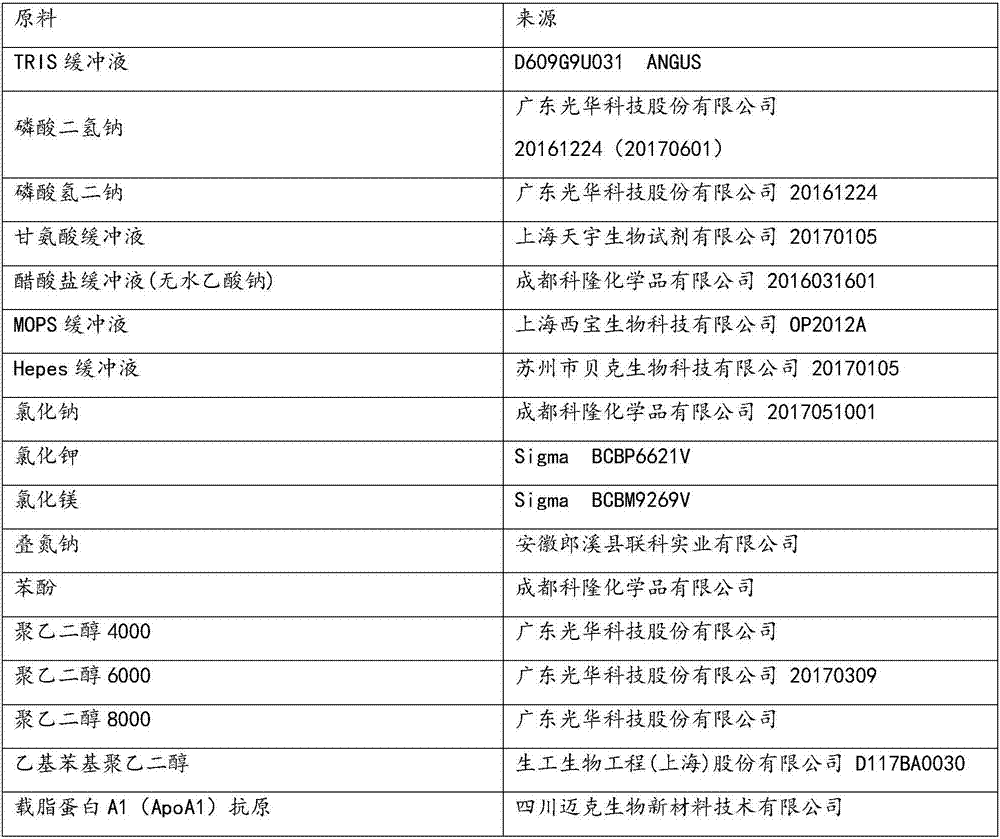
[0044] Reagent R1:
[0045]
[0046]
[0047] Reagent R2:
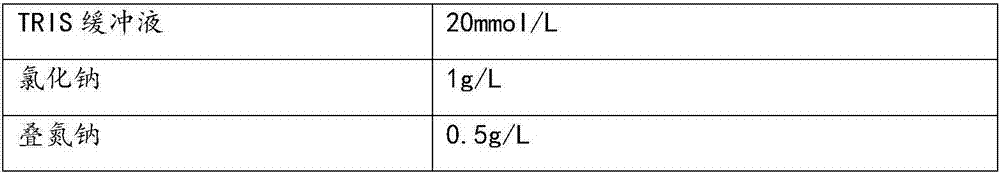
[0048] TRIS buffer
20mmol / L
1g / L
0.5g / L
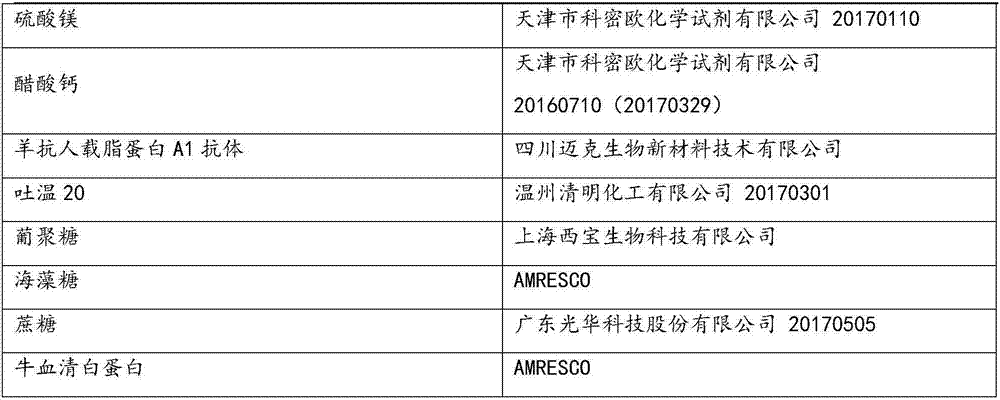
Goat anti-human apolipoprotein A1 antibody
10mg / L
Ethylphenyl polyethylene glycol
0.8g / L
0.01g / L
calcium acetate
0.01g / L
[0049] Calibrator:
[0050]ApoA1 antigen standard diluent (20mmol / L phosphate buffer, 2g / L sodium chloride, 0.1g / L sodium azide, 0.4g / L dextran, 1g / L trehalose, 1g / L sucrose, 2g / L bovine serum albumin) was dissolved, tested with a commercially available control reagent and adjusted to 120mg / L, and stored in -20°C in aliquots. Take it out before use, and dilute it into different concentrations of ApoA1 standard with standard diluent (ApoA1 antigen concentration: 0mg / L, 10mg / L, 20mg / L, 50mg / L, 70mg / L). Then use a 0.65 μm filter membrane to filter and sterilize, and store a...
Embodiment 2
[0051] Example 2 Apolipoprotein A1 detection kit
[0052] Reagent R1:
[0053] Phosphate buffer
50mmol / L
6g / L
0.7g / L
polyethylene glycol 6000
25g / L
Ethylphenyl polyethylene glycol
51g / L
[0054] Reagent R2:
[0055]
[0056]
[0057] Calibrator:
[0058] ApoA1 antigen standard diluent (50mmol / L glycine buffer, 15g / L sodium chloride, 0.1g / L sodium azide, 50g / L dextran, 25g / L trehalose, 30g / L sucrose, 20g / L L bovine serum albumin) was dissolved, tested with a commercially available control reagent and adjusted to 100 mg / L, and stored in -20°C in aliquots. Take it out before use, and dilute it into different concentrations of ApoA1 standard with standard diluent (ApoA1 antigen concentration: 0mg / L, 10mg / L, 30mg / L, 50mg / L, 70mg / L). Then use a 0.65 μm filter membrane to filter and sterilize, and store at 2-8°C.
Embodiment 3
[0059] Example 3 Apolipoprotein A1 Detection Kit
[0060] Reagent R1:
[0061] acetate buffer
90mmol / L
potassium chloride
20g / L
0.8g / L
polyethylene glycol 6000
50g / L
Ethylphenyl polyethylene glycol
5g / L
[0062] Reagent R2:
[0063] acetate buffer
90mmol / L
potassium chloride
20g / L
0.8g / L
Goat anti-human apolipoprotein A1 antibody
300mg / L
Ethylphenyl polyethylene glycol
4g / L
0.05g / L
calcium acetate
0.04g / L
[0064] Calibrator:
[0065] ApoA1 antigen standard diluent (70mmol / L TRIS buffer, 20g / L sodium chloride, 1g / L sodium azide, 70g / L dextran, 60g / L trehalose, 60g / L sucrose, 70g / L bovine serum albumin) was dissolved, tested with a commercially available control reagent and adjusted to 200mg / L, and stored in -20°C. Take it out before use, and dilute it into different concentrations of Ap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com