Fluosulfonic acid containing zwitterionic surfactant and preparation method
A surfactant and zwitterionic technology, which is applied in the field of fluorine-containing sulfonic acid zwitterionic surfactant and its preparation, can solve the problems of affecting the use effect, poor surface tension, and poor performance of fluorine-containing surfactants.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
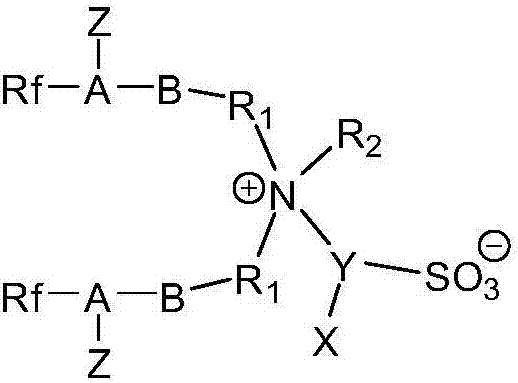
[0035] This embodiment provides a fluorine-containing sulfonic acid type zwitterionic surfactant, which has the following structure:
[0036]
[0037] The above-mentioned fluorine-containing sulfonic acid type zwitterionic surfactant provided in this example is prepared according to the following steps:
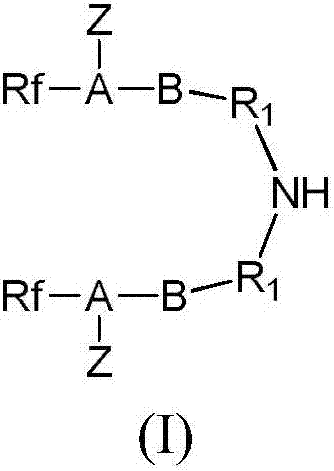
[0038] (1) Preparation of intermediate product (I): The intermediate product (I) of this example is N,N'-(imino-2,1-ethylenediyl)bisperfluoro(2,5-dimethyl-3, 6-dioxanonanoic acid)amide
[0039] Add 0.01mol (1.03g) of diethylenetriamine and 20mL of anhydrous diethyl ether into a 50mL three-necked round-bottomed flask equipped with a stirrer and a dropping funnel, and cool the reaction system with an ice-water bath. Under stirring, 0.021mol (10.81g) of perfluoro(2,5-dimethyl-3,6-dioxanonanoic acid) chloride was slowly dropped into the reaction system through the dropping funnel. After dropping, it was raised to room temperature. The reaction was stirred for another 4h. T...
Embodiment 2
[0061]This embodiment provides a fluorine-containing sulfonic acid type zwitterionic surfactant, which has the following structure:
[0062]
[0063] The above-mentioned fluorine-containing sulfonic acid type zwitterionic surfactant provided in this example is prepared according to the following steps:
[0064] (1) Preparation of intermediate product (I): N,N'-(imino-2,1-ethanediyl)bisperfluoro(2,5-dimethyl-3,6-dioxanonanoic acid)amide
[0065] Its preparation process is identical with example 1 step (1).
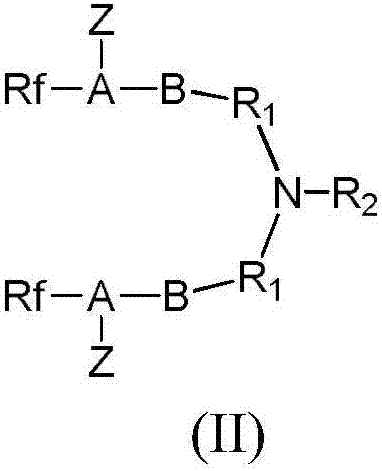
[0066] (2) Preparation of intermediate product (II): The intermediate product (II) of this example is N,N'-[(benzylimino)-2,1-ethylenediyl]bisperfluoro(2,5-dimethyl 3,6-dioxanonanoic acid)amide
[0067] Add 0.001mol (1.06g) N,N'-(imino-2,1-ethanediyl)bisperfluoro(2,5-dimethyl -3,6-dioxanonanoic acid) amide, 0.0012 mol (0.064 g) of sodium methoxide and 10 mL of anhydrous THF. Under stirring, 0.0012 mol (0.21 g) of benzyl bromide was dissolved in 5 ml of anhydrous THF,...
Embodiment 3
[0081] This embodiment provides a fluorine-containing sulfonic acid type zwitterionic surfactant, which has the following structure:
[0082]
[0083] The above-mentioned fluorine-containing sulfonic acid type zwitterionic surfactant provided in this example is prepared according to the following steps:
[0084] (1) Preparation of intermediate product (I): the intermediate product (I) of this embodiment is N, N'-(imino-2,1-ethylenediyl) bis-perfluoroheptanamide
[0085] Add 0.01mol (1.03g) of diethylenetriamine and 20mL of anhydrous THF into a 50mL three-necked round-bottomed flask equipped with a stirrer and a dropping funnel, and cool the reaction system with an ice-water bath. Under stirring, 0.022mol (8.32g) of methyl perfluoroheptanoate was slowly dropped into the reaction system through the dropping funnel. After dropping, it was raised to room temperature. The reaction was stirred for another 4h. GC tracks the reaction endpoint. After the reaction was complete, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com