Method for preparing ethylbenzene by utilizing light alkanes
A technology for low-carbon alkanes and ethylbenzene, which is applied in the field of preparing ethylbenzene, can solve the problems of difficulty in ethylene transportation, improve the entry threshold of the ethylbenzene market, etc., and achieve the effects of flexible device design scale, compact production space design, and efficient utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Using the low-value product saturated liquefied gas LPG in the refinery as raw material to produce 100,000 tons / year of ethylbenzene.
[0046] The composition of the raw materials used in this embodiment is shown in Table 1 below:
[0047] Table 1
[0048] composition
[0049] The raw material consumption of present embodiment is as shown in table 2 below:
[0050] Table 2
[0051] raw material
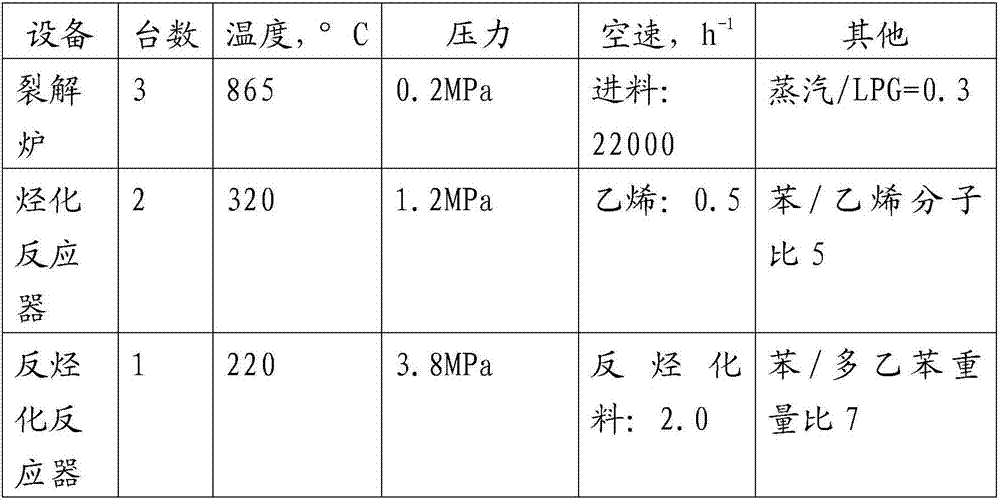
[0052] The main processing conditions of the present embodiment are shown in table 3 below:
[0053] table 3
[0054]
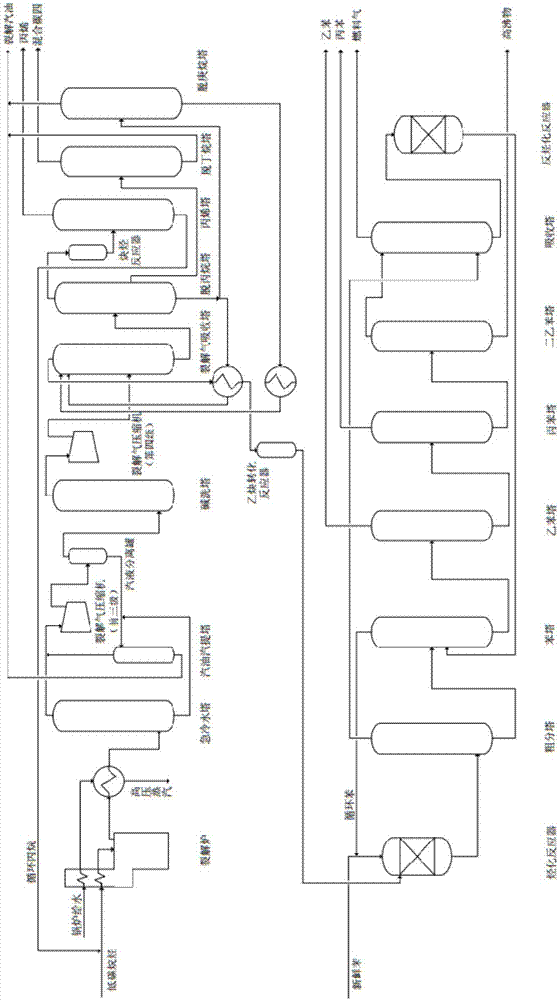
[0055] The technology that present embodiment produces ethylbenzene is as follows:
[0056] (1) cracking unit:
[0057] The normal temperature saturated liquefied gas with a pressure of about 1.5MPa (in the continuous production process, the saturated liquefied gas can be combined with the circulating propane produced in the subsequent steps) is preheated by quenching water and then enters the vaporizer, and is fully vaporized befor...
Embodiment 2
[0088] In this embodiment, n-butane is selected as raw material to produce 300,000 tons / year of ethylbenzene. For the process of this embodiment, refer to Embodiment 1, and details are not repeated here.
[0089] The composition of the raw materials used in this embodiment is shown in Table 5:
[0090] table 5
[0091] composition
wt%
n-butane
100
total
100
[0092] The raw material consumption of this embodiment is shown in Table 6.
[0093] Table 6
[0094] raw material
KTA
kg / hr
n-butane
195
24392
219
27381
total
414
51772
[0095] The product distribution of this embodiment is shown in Table 7.
[0096] Table 7
[0097] product
KTA
kg / hr
58
7227
Propylene
36
4442
mixed carbon tetraolefins
11
1323
of which butadiene
7
866
6
768
300...
Embodiment 3
[0104] In this embodiment, ethane is selected as a raw material to produce 600,000 tons / year of ethylbenzene. For the process of this embodiment, refer to Embodiment 1, and details are not repeated here.
[0105] The composition of raw materials in this example is shown in Table 9.
[0106] Table 9
[0107] composition
wt%
1.26
ethane
93.77
propane
4.97
total
100
[0108] See Table 10 for the consumption of raw materials in this embodiment.
[0109] Table 10
[0110] raw material
KTA
kg / hr
ethane
311
38860
benzene
448
55946
total
758
94806
[0111] The product distribution of this embodiment is shown in Table 11.
[0112] Table 11
[0113]
[0114] Propane / butane recycle, and recovery of benzene from pyrolysis gasoline are considered in Table 11.
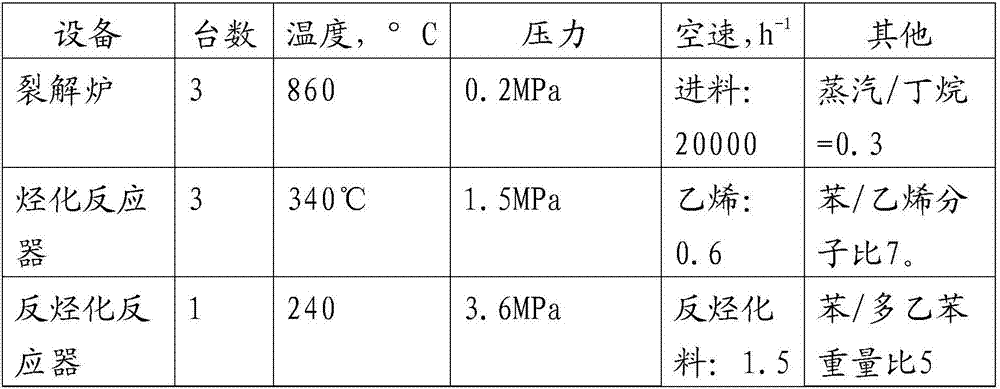
[0115] The main process conditions of this embodiment are shown in Table 12.
[0116] Table 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com