Composite material for improving reaction activity of Fe(III)-TAML and preparation method and application method thereof
A technology based on hexamethylphenyl and composite materials, which can be used in chemical instruments and methods, other chemical processes, organic compounds/hydrides/coordination complex catalysts, etc., and can solve the problems of high synthesis cost and low yield. , to achieve the effect of improving the reactivity, improving the reactivity and promoting the ionization of water molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
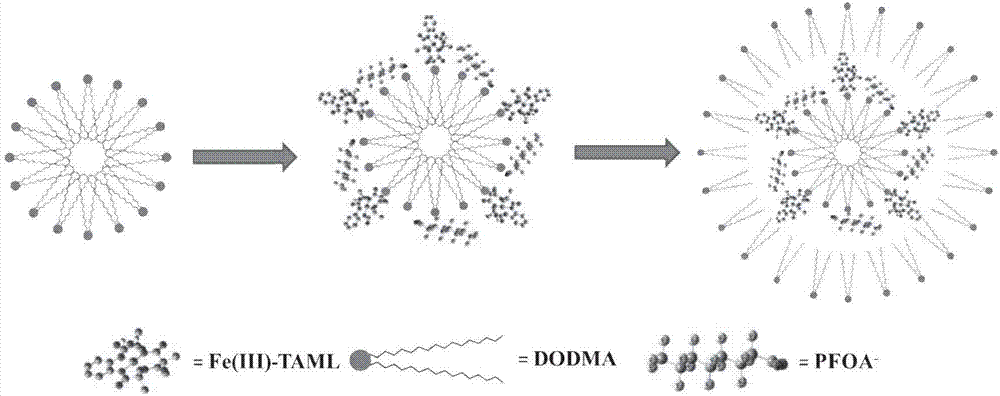
[0045] The preparation method of the composite material for improving the reaction activity of tetraamidohexamethylphenyl ring iron of the present invention comprises the following steps:
[0046] (1) DODMA is dissolved in methylene chloride;
[0047] (2) Dissolving Fe(III)-TAML in ultrapure water;
[0048] (3) Dissolve PFOA in ultrapure water, adjust the pH of the solution to 6-8;
[0049] (4) adding the PFOA in step (3) to the Fe(III)-TAML solution in step (2) to obtain a mixed solution of Fe(III)-TAML and PFOA;
[0050] (5) DODMA, Fe(III)-TAML and PFOA are mixed in a molar ratio of 1:(3-12):(0.01-1);
[0051] (6) after adding the DODMA solution dropwise to the mixed solution of Fe(III)-TAML and PFOA, use a magnetic stirrer to stir for 24 hours to ensure that dichloromethane is completely volatilized;
[0052] (7) After the stirring is completed, let it stand for at least 24 hours, so that the composite material Fe(III)-TAML / DODMA / PFOA can be fully aged.
[0053] In the ...
Embodiment 1
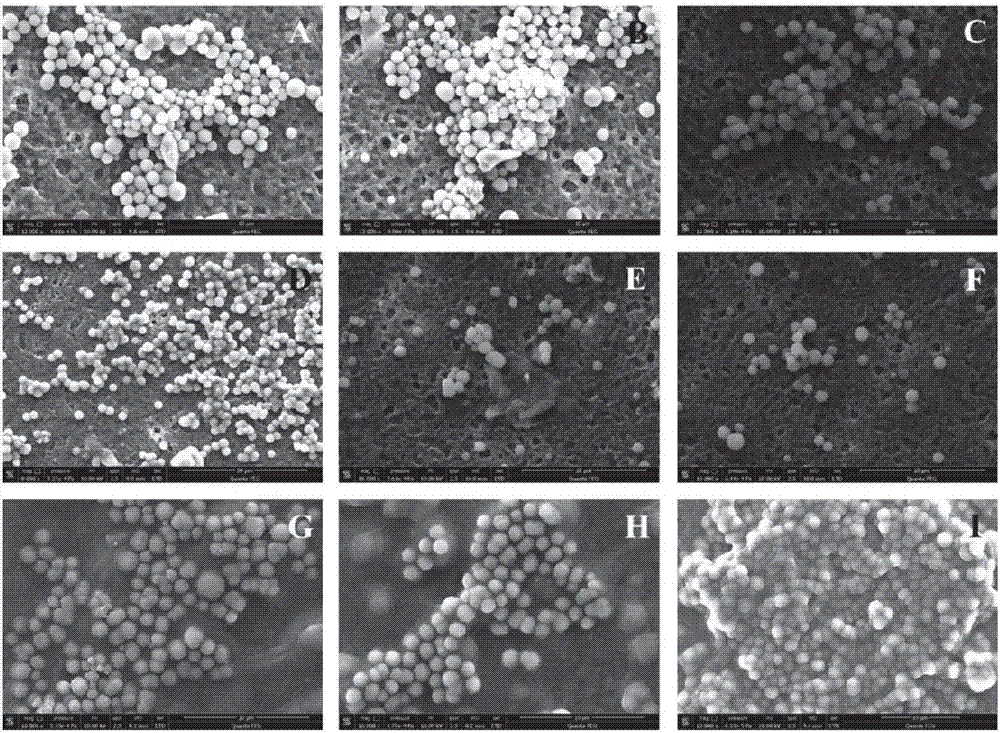
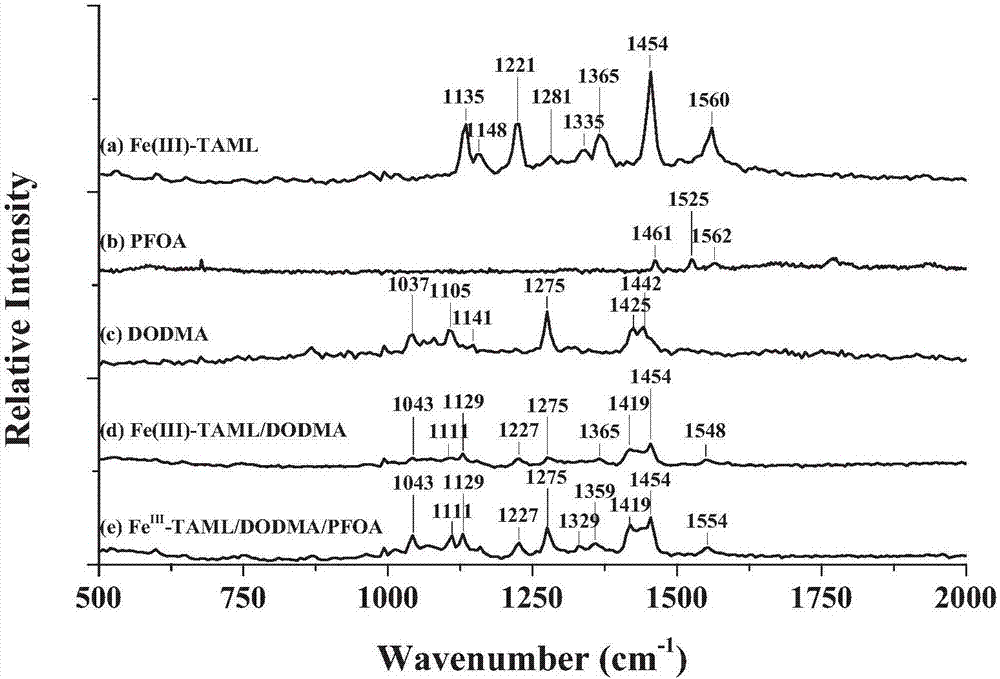
[0061] A composite material preparation method for improving the reactivity of tetraamidohexamethylphenyl ring iron, comprising the steps of: using a surfactant-assisted self-assembly method to dissolve dioctadecyldimethyl chloride in dichloromethane Ammonium DODMA (30 mM) was added dropwise to 20 mL of a mixed aqueous solution of Fe(III)-TAML (100 μM) and PFOA (1, 5, 10, 50, and 100 μM) to synthesize Fe(III)-TAML-based composites Fe(III)-TAML / DODMA / PFOA; wherein the molar ratio of Fe(III)-TAML, DODMA and PFOA is 1:3:0.01, 1:3:0.1, 1:3:1, 1:6:0.01, 1:6:0.1, 1:6:1, 1:12:0.01, 1:12:0.1, 1:12:1. During the preparation process, for all treatment groups, a yellow opaque emulsion was formed when DODMA was added, and Fe(III)-TAML in the solution settled to the bottom of the solution after standing for separation; After stirring for more than 24 hours, a stable yellow suspension was obtained, which was then used for characterization, degradation and stability experiments after standi...
Embodiment 2
[0064] A composite material preparation method for improving the reactivity of tetraamidohexamethylphenyl ring iron, comprising the steps of: using a surfactant-assisted self-assembly method to dissolve dioctadecyldimethyl chloride in dichloromethane Ammonium DODMA (30 mM) was added dropwise to 20 mL of a mixed aqueous solution of Fe(III)-TAML (100 μM) and PFOA (1, 5, 10, 50, and 100 μM) to synthesize Fe(III)-TAML-based composites Fe(III)-TAML / DODMA / PFOA. The molar ratios of Fe(III)-TAML, DODMA and PFOA were 1:3:0.01, 1:3:0.1, 1:3:1, 1:6:0.01, 1:6:0.1, 1:6:1, 1:12:0.01, 1:12:0.1, 1:12:1. During the preparation process, for all treatment groups, a yellow opaque emulsion was formed when DODMA was added, and Fe(III)-TAML in the solution settled to the bottom of the solution after standing for separation. When vigorously stirred for more than 24 hours, a stable yellow suspension was obtained, which was then used for characterization, degradation and stability experiments after s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com