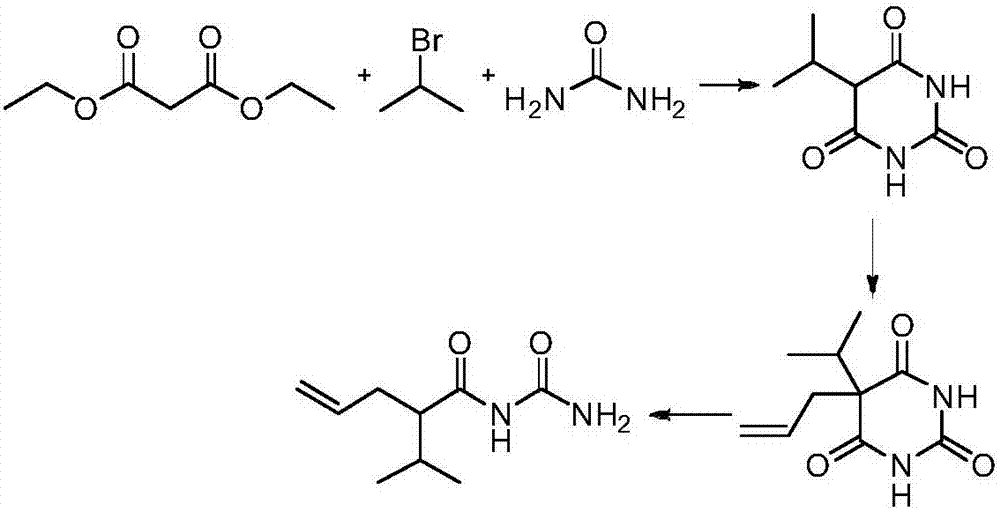
Synthesis method of apronal
A technology of valproic acid urea and synthesis method, which is applied in chemical instruments and methods, preparation of urea derivatives, preparation of organic compounds, etc., can solve the problems of using many kinds of raw materials, consuming more energy, increasing costs, etc., and achieve the synthesis route Simple and efficient, low cost and high raw material utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
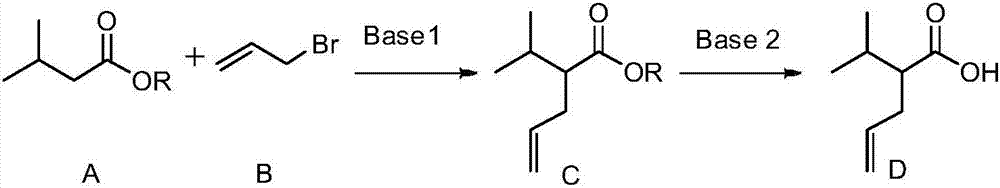
[0029] 1. Synthesis of 2-isopropyl-4-enpentanoic acid
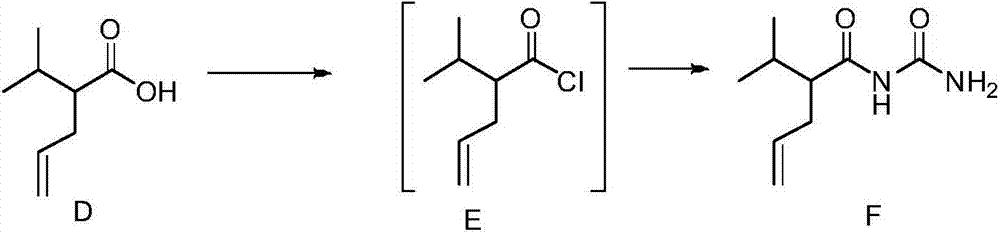
[0030] Add 15 mL of tetrahydrofuran and 10 mL of 2.0 mol / L lithium diisopropylamide in tetrahydrofuran to a 100 mL Reyk bottle, and then add 1.1 mL (10.0 mmol) of isovaleric acid represented by formula A-1 dropwise at -15°C, React for 1 hour, then add 1.04mL (12.0mmol) allyl bromide, return to room temperature naturally, continue the reaction for 1 hour, adjust the pH value to 1-2 with 10% hydrochloric acid, add 20mL petroleum ether for extraction, and then Wash with 15 mL of 10% hydrochloric acid, water and brine, combine the organic phases, add anhydrous sodium sulfate to dry, and remove the organic solvent under reduced pressure to obtain 1.2 g of 2-isopropyl-4-enpentanoic acid represented by formula D , the yield is 86%, and the structural characterization data are as follows: 1 H NMR (600MHz, CDCl 3 )δ5.71(td,J 1 =17.0,6.9Hz,1H),4.98(dd,J 1 =38.7,J 2 =13.6Hz, 2H), 2.32-2.22(m, 2H), 2.20-2.13(m, 1H), ...
Embodiment 2
[0034]
[0035] 1. Synthesis of 2-isopropyl-4-enpentanoic acid
[0036]Dissolve 0.9g (7.7mmol) of potassium tert-butoxide in 15mL of tetrahydrofuran, stir at -78°C for 20 minutes, then add 1.2mL (7.7mmol) of ethyl isovalerate represented by formula A-2 dropwise into the reaction system , reacted at -78°C for 2.5 hours, then added 0.6mL (8.1mmol) of allyl bromide represented by formula B dropwise into the reaction system, continued to react at -78°C for 2.5 hours, and removed the organic solvent under reduced pressure to obtain Add 20 mL of ethanol and 55 mL of 3mol / L NaOH aqueous solution to the 2-isopropyl-4-valerate ethyl ester shown in formula C-2, react at 55°C for 12 hours, pour the reaction solution into 30 mL of 10% glacial hydrochloric acid, then extracted twice with 50mL of petroleum ether, combined the organic phases, and washed them with 15mL of 10% hydrochloric acid, water and brine respectively, combined the organic phases, added anhydrous sodium sulfate to dry...
Embodiment 3
[0040] Potassium tert-butoxide in Step 1 of Example 2 is replaced by equimolar lithium diisopropylamide, other steps are the same as in Example 2, the yield of 2-isopropyl-4-valerate is 80%, and the rest The steps and yields are the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com