Preparation method of bupivacaine hydrochloride
A technology of bupivacaine hydrochloride and potassium carbonate, which is applied in the field of preparation of bupivacaine hydrochloride, can solve the problems of long reaction time, low reaction yield and high production cost, and achieves high product quality, low operating cost and stable operation. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A kind of preparation method of bupivacaine hydrochloride of invention comprises the steps:
[0021] (1) Preparation of N-(2,6-xylyl)-2-piperidinecarboxamide:
[0022]
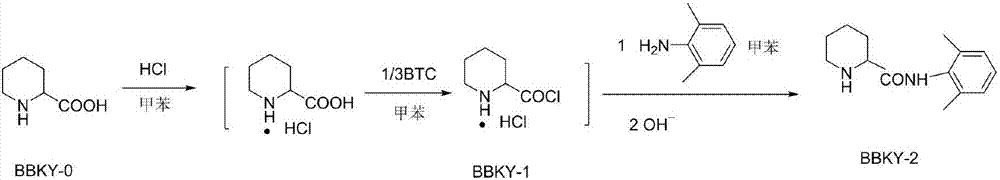
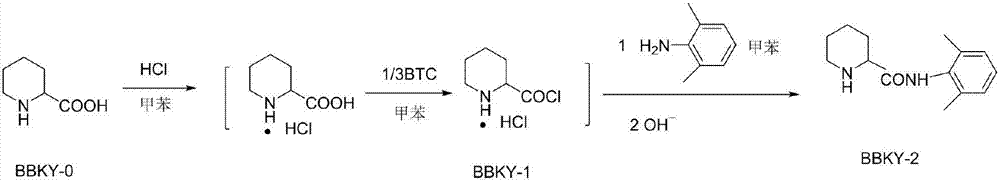
[0023] Add 2-piperidinecarboxylic acid into a three-necked flask, pass hydrogen chloride gas through toluene at room temperature for 1 hour, heat the mixture to 55°C, add the toluene solution of bis(trichloromethyl)carbonate BTC dropwise within 2 hours, and keep the temperature for reaction For 5 hours, add a toluene solution of 2,6-dimethylaniline dropwise, react at 55°C for 2 hours, filter, wash with toluene, dissolve the filter cake in water, adjust the pH to 5.5 with 20% NaOH, extract with toluene, and recover the organic 2,6-dimethylaniline in the phase, adjust the water phase to pH 11, extract with toluene, separate layers, and evaporate the solvent to obtain a white solid, which is then washed with petroleum ether, filtered, and dried to obtain N-(2, 6-xylyl)-2-piperidinecarboxamide BBKY-2; ...
Embodiment 2
[0031] The difference between embodiment 2 and embodiment 1 is: a kind of preparation method of bupivacaine hydrochloride of invention, comprises the steps:
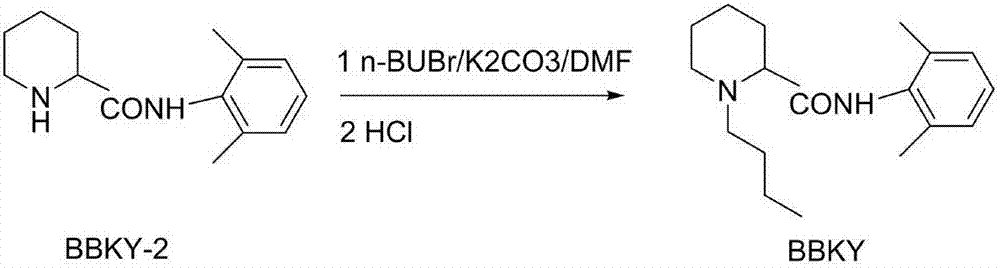
[0032] In step (1), 6.5 g of 2-piperidinecarboxylic acid was added to a three-necked flask, 100 ml of toluene was passed through hydrogen chloride gas at room temperature for 1 hour, the mixture was heated to 55° C., and 5 g of bis(tri) was added dropwise within 2 hours. Chloromethyl)carbonate BTC toluene (20ml) solution, keep warm for 5 hours, add 2,6-dimethylaniline 32.5ml toluene (10ml) solution dropwise, react at 60°C for 2 hours; filter, wash with toluene, filter Dissolve the cake in water, adjust the pH to 4.5 with 20% NaOH, extract with toluene, and recover 26ml of 2,6-dimethylaniline in the organic phase; adjust the pH of the aqueous phase to 11.5, extract with toluene, separate layers, and evaporate solvent to obtain a white solid; then washed with petroleum ether, filtered, and dried to obtain 5.8 g of N-(2,6-x...
Embodiment 3
[0034] The difference between embodiment 3 and embodiment 1 is:
[0035] A kind of preparation method of bupivacaine hydrochloride of the present invention comprises the steps:
[0036] In step (1), 6.5 g of 2-piperidinecarboxylic acid was added to a three-necked flask, 100 ml of toluene was passed through hydrogen chloride gas at room temperature for 1 hour, the mixture was heated to 58 ° C, and 5 g of bis(tri Chloromethyl)carbonate BTC in toluene (20ml) solution, keep warm for 5 hours, add dropwise 2,6-dimethylaniline 32.5ml in toluene (10ml) solution, react at 55-60°C for 2 hours; filter, wash with toluene , the filter cake was dissolved in water, adjusted to pH 4.8 with 20% NaOH, extracted with toluene, and 26ml of 2,6-dimethylaniline in the organic phase was recovered; the aqueous phase was adjusted to pH 12, extracted with toluene, and separated. The solvent was evaporated to obtain a white solid; it was washed with petroleum ether, filtered, and dried to obtain 5.8 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com