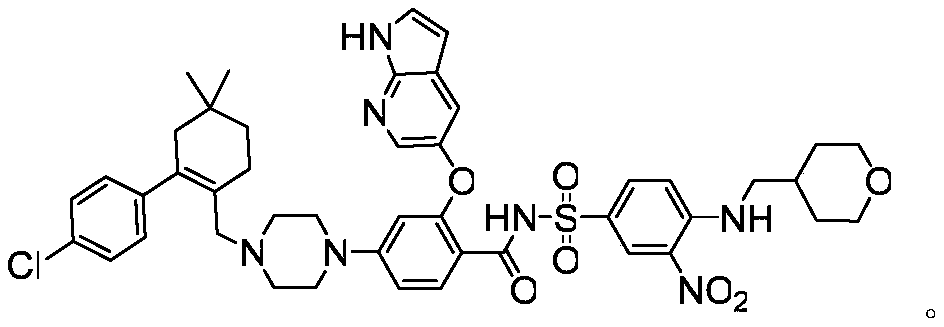
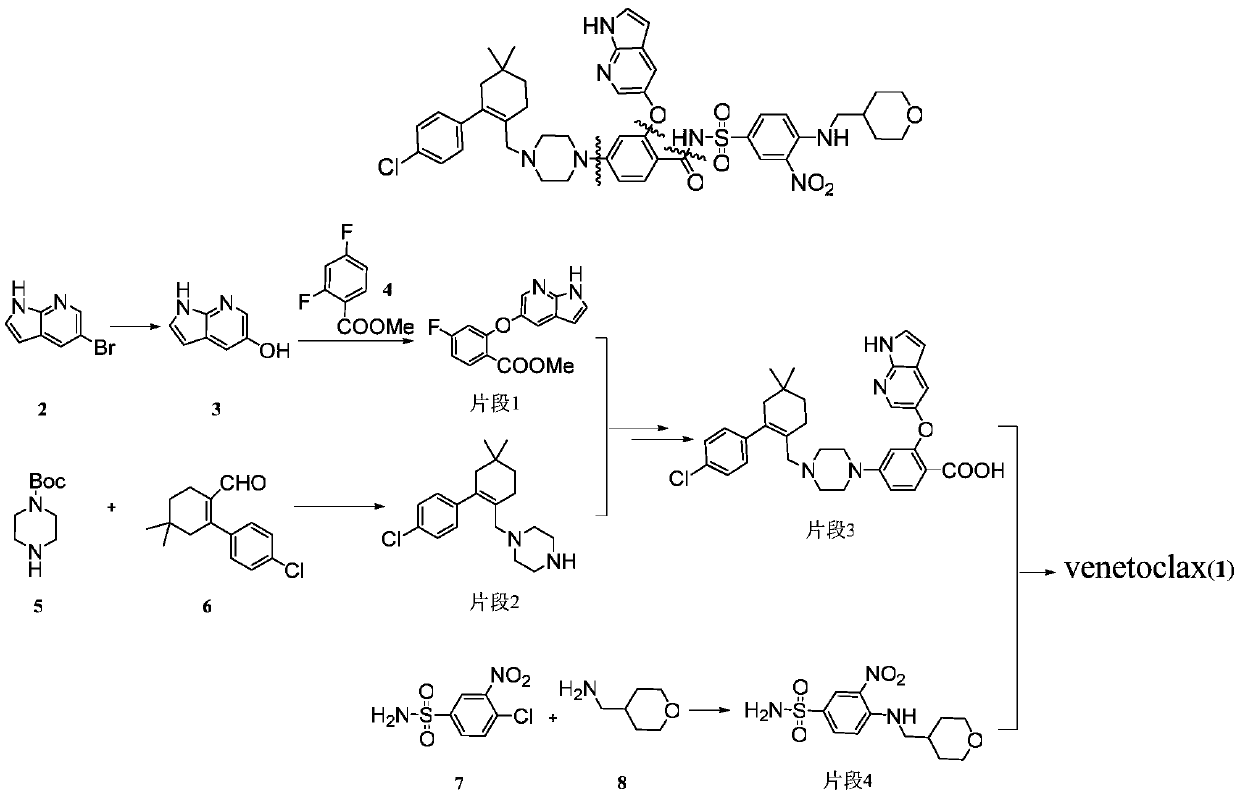
A kind of preparation method of bcl-2 inhibitor venetoclax and intermediate
A technology of bcl-2 and intermediates, applied in the field of drug synthesis, to achieve the effects of reducing synthesis costs, simple reaction operations, and saving raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
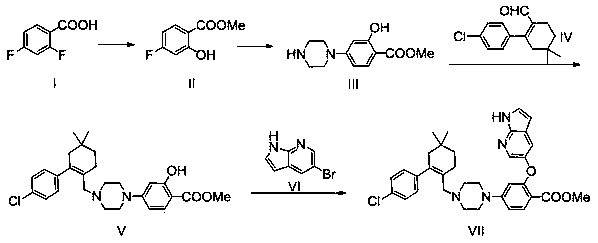
[0030] Example 1: Preparation of methyl 4-fluorosalicylate (compound II)
[0031]
[0032] The preparation of methyl 4-fluorosalicylate (II) involves two steps.
[0033] Step 1: Preparation of 4-fluorosalicylic acid
[0034]Dimethylsulfoxide (DMSO, 4L) was added to a dry 10L four-necked flask, 2,4-difluorobenzoic acid (500g, 3.16mol) and NaOH (253g, 6.32mol) were added under stirring, and the temperature was raised to 130°C, reacted for about 8 hours, and detected by TLC. After the reaction was complete, the reaction system dropped to room temperature, and the reaction solution was slowly poured into 40L of ice water, and the pH was adjusted to 2-3 with concentrated hydrochloric acid (the temperature during the pH adjustment process should not be higher than 20°C), a large amount of solids were precipitated, continued to stir for 2h and then suction filtered, the filter cake was washed with an appropriate amount of water to obtain a white solid, and dried under reduced pre...
Embodiment 2
[0037] Embodiment two: the preparation of compound III
[0038]
[0039] Add DMSO (5L) into a dry 10L four-neck flask, add compound II (400g, 2.35mol) under stirring, slowly add piperazine (607g, 7.05mol), react at room temperature for 5h, and detect by TLC. After the reaction is complete, the The reaction solution was slowly poured into 40L of ice water, extracted with ethyl acetate (20L×3), the ethyl acetate was combined, and the organic phase was washed three times with saturated brine (10L×3), dried and concentrated under reduced pressure to obtain 500g of compound III , The crude yield is 90%. It was directly used in the next reaction without purification.
Embodiment 3
[0040] Embodiment three: the preparation of compound V
[0041]
[0042] Add dichloromethane (7L) into a 10L four-neck flask, add compound III (500g, 2.11mol) and raw material IV (577g, 2.32mol) under stirring, and slowly add sodium triacetoxyborohydride (352g, 1.67 mol), temperature control is not higher than 30 °C, after addition, react at room temperature for 5 hours, TLC monitoring, after the reaction is complete, add saturated saline (1.5L×2) to wash twice, the organic phase is dried with anhydrous magnesium sulfate, and suction filtered , concentrated under reduced pressure, dissolved the concentrate in ethyl acetate (1L), and heated to 50°C to dissolve, then slowly added n-hexane (5L) dropwise, solids gradually precipitated, kept at 10-15°C and stirred for 2h, then suction filtered , the filter cake was washed with a mixed solvent (0.5 L) of ethyl acetate and n-hexane (volume ratio 1:3), and air-dried at 50° C. to obtain 794 g of an off-white solid, with a yield of 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com