Novel carboxymethyl chitosan derivative, and preparation method and application thereof
A technology of carboxymethyl chitosan and its derivatives, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Drug insoluble and other problems, to achieve the effect of uniform shape, uniform size, uniform particle size dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation method of carboxymethyl chitosan-vitamin E succinate:
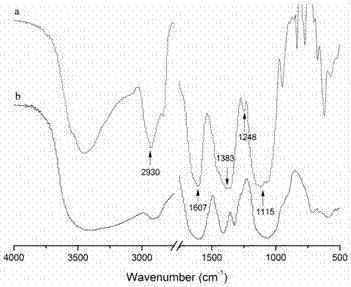
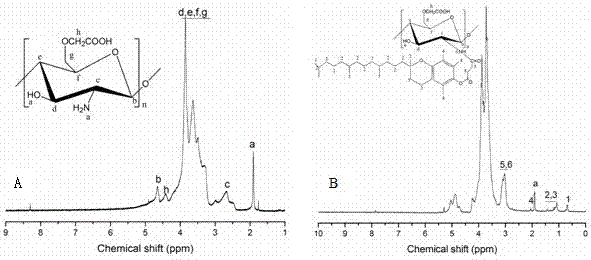
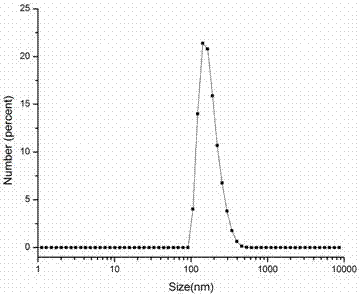
[0032] Weigh 0.66g of O-carboxymethyl chitosan (O-CMCTS) and dissolve it in 60mL of deionized water to obtain an aqueous solution of carboxymethyl chitosan; weigh 3.16g of vitamin E succinate (VES) and dissolve it in 70mL In dimethylformamide (DMF), after dissolving, add 1.16g EDC and 0.69g NHS, after catalyzing 30min, the carboxymethyl chitosan aqueous solution of above-mentioned preparation is dripped in this reaction solution, after dropwise adding, At room temperature, the reaction was mechanically stirred at a speed of 200 rpm for 48 h. After the reaction was completed, the reaction solution was precipitated with 5 times the volume (650 mL) of absolute ethanol, and after suction filtration, the precipitate was washed three times with an appropriate amount of absolute ethanol, then dissolved in deionized water, dialyzed for three days, and freeze-dried to obtain carboxymethyl Chit...
Embodiment 2
[0033] Embodiment 2: the preparation method of carboxymethyl chitosan-vitamin E succinate:
[0034] Weigh 0.66g of O-carboxymethyl chitosan (O-CMCTS) and dissolve it in 60mL deionized water to obtain an aqueous solution of carboxymethyl chitosan; weigh 1.59g vitamin E succinate (VES) and dissolve it in 70mL In dimethylformamide (DMF), after dissolving, add 0.58g EDC and 0.35g NHS to catalyze for 30min, drop the carboxymethyl chitosan aqueous solution prepared above into the reaction solution drop by drop, Under these conditions, the reaction was mechanically stirred at a speed of 200 rpm for 48 h. After the reaction was completed, the reaction solution was precipitated with 5 times the volume (650 mL) of absolute ethanol, and after suction filtration, the precipitate was washed three times with an appropriate amount of absolute ethanol, then dissolved in deionized water, dialyzed for 3 days, and freeze-dried to obtain carboxymethyl Chitosan-Vitamin E Succinate. According to ...
Embodiment 3
[0035] Embodiment 3: the preparation method of carboxymethyl chitosan-vitamin E succinate:
[0036]Weigh 0.66g of O-carboxymethyl chitosan (O-CMCTS) and dissolve it in 60mL of deionized water to obtain an aqueous solution of carboxymethyl chitosan; weigh 1.19g of vitamin E succinate (VES) and dissolve it in 70mL In dimethylformamide (DMF), after dissolving, add 0.47g EDC and 0.26g NHS to catalyze for 30min, then drop the carboxymethyl chitosan aqueous solution prepared above into the reaction solution drop by drop, Under these conditions, the reaction was mechanically stirred at a speed of 200 rpm for 48 h. After the reaction was completed, the reaction solution was precipitated with 5 times the volume (650 mL) of absolute ethanol, and after suction filtration, the precipitate was washed three times with an appropriate amount of absolute ethanol, then dissolved in deionized water, dialyzed for 3 days, and freeze-dried to obtain carboxymethyl Chitosan-Vitamin E Succinate. Acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com