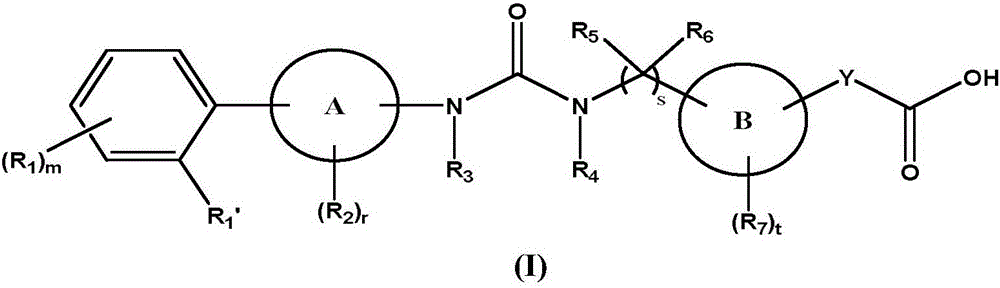
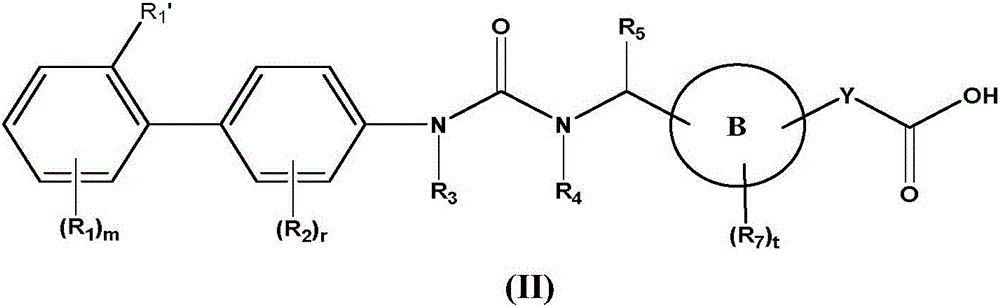
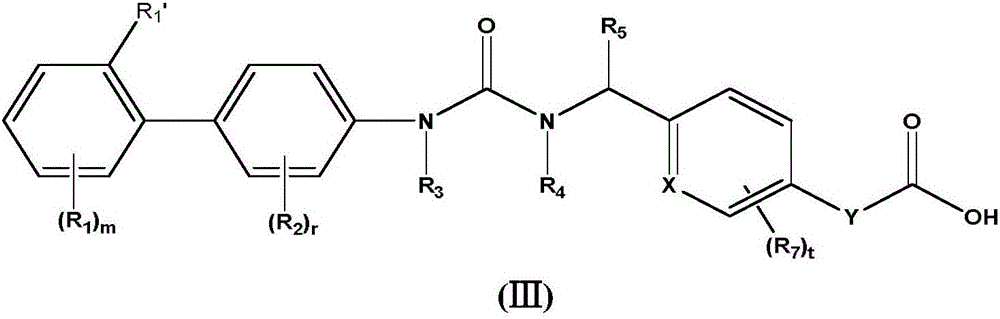
Biaryl urea carboxylic acid derivative or salt as well as preparation method and application thereof
A technology of carboxylic acid derivatives and biaryl urea, which is applied in the field of biaryl urea carboxylic acid derivatives and their preparation, and can solve the problems of reduced ability of differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: 4-((3-(2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido)methyl)benzene formic acid
[0099] (4-((3-(2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido)methyl)benzoic acid)
[0100]
[0101] Step 1: 4-((3-(2,6-Dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido)methyl)benzoic acid methyl ester
[0102] Add 2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-amine (100mg, 0.31mmol), DCM (5mL) to a 25mL single-necked bottle, DIEA (120mg, 0.93mmol) was stirred in an ice bath for 5 minutes, then triphosgene (33mg, 0.11mmol) was added, and the ice bath reaction was continued for 30 minutes, then methyl 4-aminomethylbenzoate (56mg, 0.34mmol) was added to continue the reaction on ice Bath for 30 minutes, then react overnight at room temperature. Join H 2 O (10mL), extracted with dichloromethane (10mLx3), combined the organic layers, washed with saturated sodium chloride (10mL), dried over anhydrous sodium sulfate, filtered, a...
Embodiment 2
[0105] Example 2: 2-(4-((3-(2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido)methanol base) phenyl) acetic acid
[0106] (2-(4-((3-(2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido)methyl)phenyl)acetic acid)
[0107]
[0108] Step 1: 2-Bromo-1,3-dichloro-5-nitrobenzene
[0109]Add 2,6-dichloro-4-nitroaniline (5g, 24mmol), copper bromide (16g, 72mmol) and acetonitrile (50mL) into a single-necked bottle, add tert-butyl nitrite dropwise under stirring in an ice bath ( 7.46g, 72mmol), then stirred and reacted at room temperature for 6 hours, after the reaction was completed, water (100ml) was added, extracted with ethyl acetate (100mLx2), saturated sodium chloride (100mL), dried over anhydrous sodium sulfate, concentrated under pressure to obtain orange 6.3 g of solid, 97% yield.
[0110] Step 2: 4-Bromo-3,5-dichloroaniline
[0111] At room temperature, add 2-bromo-1,3-dichloro-5-nitrobenzene (1g, 4mmol), ethanol (6mL), tetrahydrofuran (1mL), con...
Embodiment 3
[0118] Example 3: 2-(4-((3-(2-chloro-6-cyano-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido )methyl)phenyl)acetic acid
[0119] (2-(4-((3-(2-chloro-6-cyano-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)ureido)methyl)phenyl)acetic acid)
[0120]
[0121] Step 1: 3-Chloro-5-cyano-2-bromobenzene
[0122] Add 2-amino-3-chloro-5-nitrobenzonitrile (3g, 15mmol), copper bromide (4g, 18mmol), acetonitrile (50mL) into a 25mL single-necked bottle, stir in an ice bath for 5 minutes, and weigh Amyl nitrite (6.76g, 57.8mmol) in acetonitrile (20mL) was added dropwise to the reaction solution under ice-cooling, and then continued to react in ice-bath for 30 minutes, then naturally warmed to room temperature, and reacted overnight. Add water (100mL), extract with ethyl acetate (100mLx3), wash with water (100mL), wash with saturated sodium chloride (100mL), dry over anhydrous sodium sulfate, and spin to dry the solvent. The crude product is separated on a silica gel column to obtain a yell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com