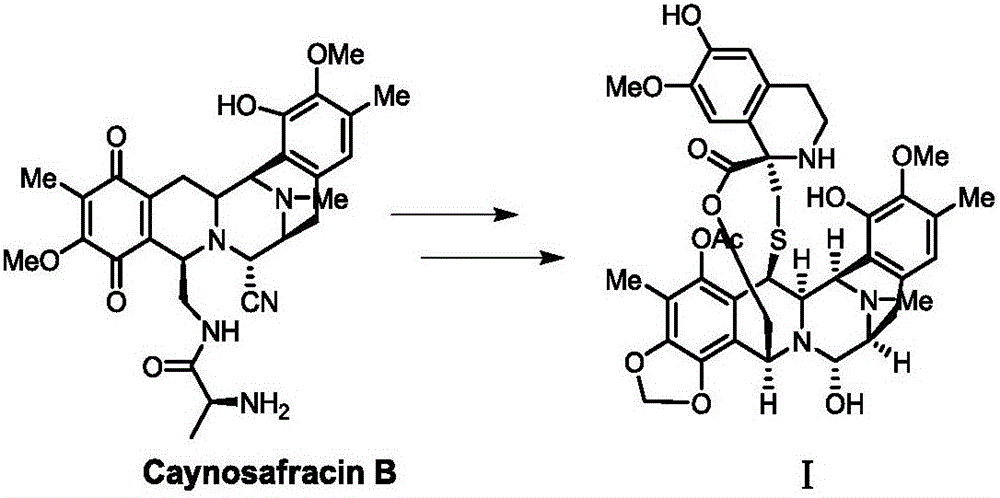
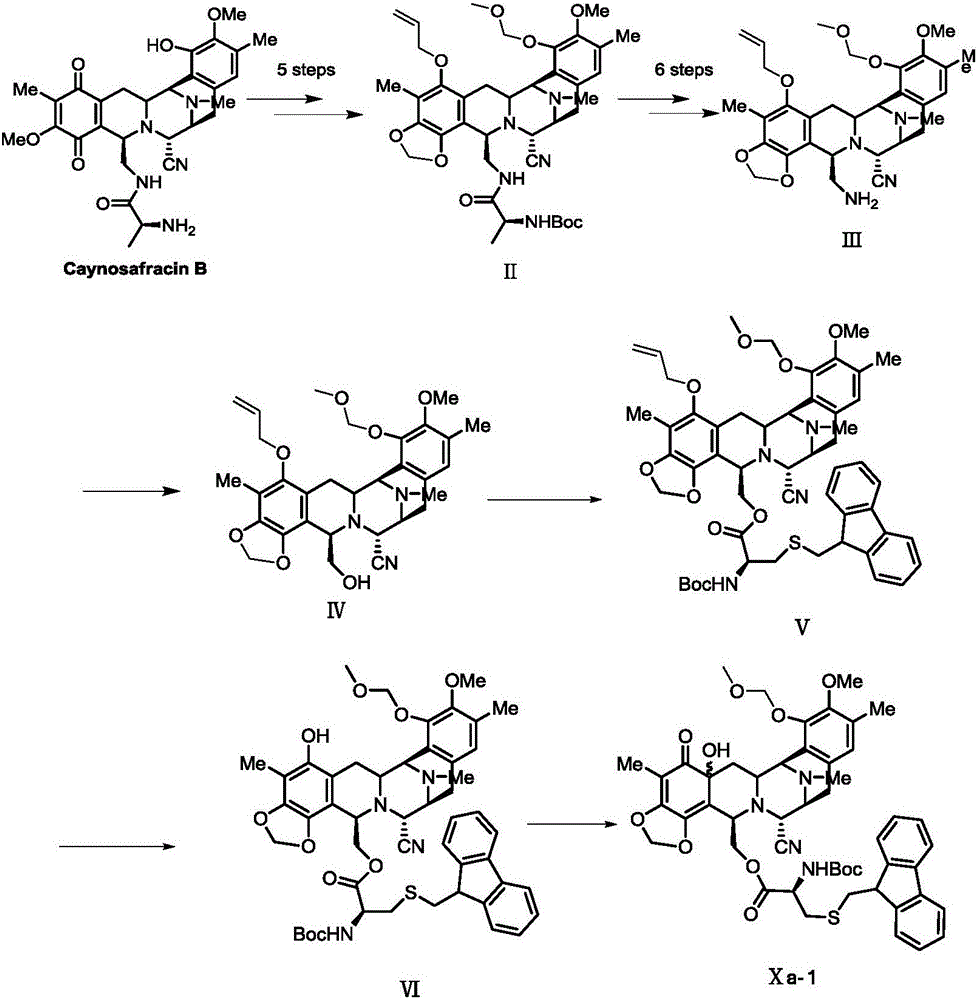
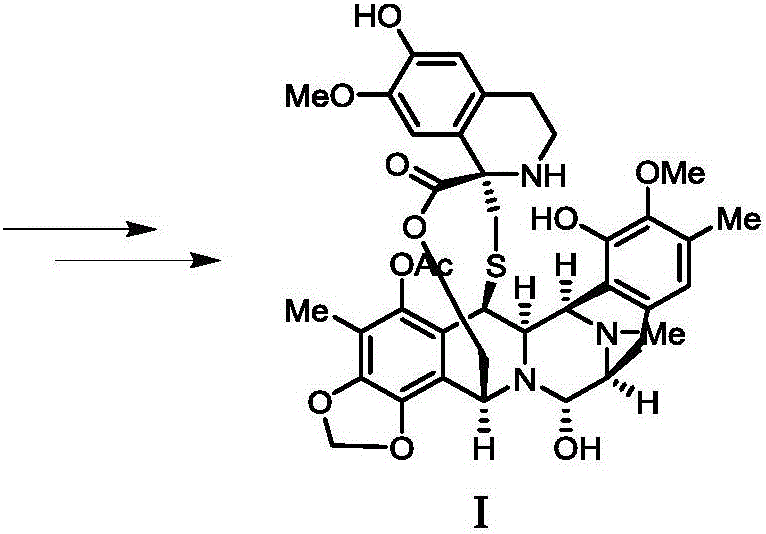
Preparation method and intermediate of trabectedin
A technology of compound and hydroxyl protecting group, applied in the field of chemistry, can solve the problems of high cost, harsh reaction conditions, long route, etc., achieve the effect of large industrial application value and simplified synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: preparation compound IIa-1
[0098]
[0099] Add Safracin B (51g) into the reaction flask at room temperature, add dichloromethane (100ml) to dissolve, add dropwise phenylisothiocyanate (76g), stir for 24h, concentrate to remove dichloromethane, and obtain a brown oil 150g. (No purification and next step reaction)
[0100] 1H NMR (400MHZ, DMSO) δ: 9.94(s, 1H), 8.66(s, 1H), 7.46-7.40(m, 3H), 7.36(t, J=8Hz, 3H), 7.15(t, J=7.6Hz ,1H),6.20(s,1H),4.47(s,1H),4.36(t,J=7.2Hz,1H),3.99-3.63(m,5H),3.52(s,1H),3.33(s, 3H), 2.99(d, J=2.8Hz, 1H), 2.96-2.73(m, 4H), 2.16(s, 3H), 1.98(s, 3H), 1.84(s, 3H), 1.59(m, 1H ), 1.15(d, J=6.1Hz, 2H), 0.46(d, J=2.8Hz, 3H).MS: m / z(675), Found: 658(M-H 2 O+H)
Embodiment 2
[0101] Embodiment 2: preparation compound IIIa-1
[0102]
[0103] Add tetrahydrofuran (250ml) and acetic acid (56ml) to the oil obtained in Example 1, cool down to -10°C, add dropwise an aqueous solution of sodium cyanide (12g sodium cyanide, 100ml water) with stirring, and react for 0.5h . Add saturated sodium carbonate to adjust the pH to 10, add ethyl acetate (200ml), separate the layers, wash the organic layer twice with saturated brine, dry the organic layer with anhydrous sodium sulfate, filter with suction, concentrate to obtain an oily substance, column chromatography (petroleum Ether: ethyl acetate = 4:1, 3:1, 2:1, 1:1) to obtain 54.9 g of a yellow solid with a yield of 85%.
[0104] 1 H NMR (400MHZ, DMSO) δ: 9.87(s, 1H), 8.55(s, 1H), 7.45-7.43(m, 3H), 7.36-7.32(m, 2H), 7.15-7.05(m, 2H), 6.22 (s,1H),4.88(d,J=5.2Hz 1H),4.42(m,1H),4.39(m,1H),4.28(m,1H),4.13(s,1H),3.90(d,J =2.4Hz,1H),3.89(s,3H),3.87(m,1H),3.53(m,4H),3.11-2.96(m,3H),2.85-2.55(m,3H),2.11(s, 3H), 2...
Embodiment 3
[0105] Embodiment 3: preparation compound IVa-1
[0106]
[0107] Take compound Ⅲa-1 (25g), add 70ml of methanol, cool down to 0°C in an ice bath, add trimethylchlorosilane (25ml) dropwise, after the addition is complete, keep the reaction for 3h, filter the filter cake with dichloromethane (20ml×2 ) was washed and dried to obtain 13.8 g of a yellow solid with a yield of 92%.
[0108] 1 H NMR (400MHz, DMSO) δ9.35(s,1H),7.53(s,3H),6.52(s,1H),5.06(s,1H),4.53(s,2H),4.14(d,J= 14.6Hz,3H),3.97(s,4H),3.64(s,3H),3.28(s,1H),3.19–2.84(m,5H),2.20(s,3H),1.85(s,3H), 1.74 (dt, J=23.8, 11.9Hz, 1H). MS: m / z (514), Found: 479 (M-Cl)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com