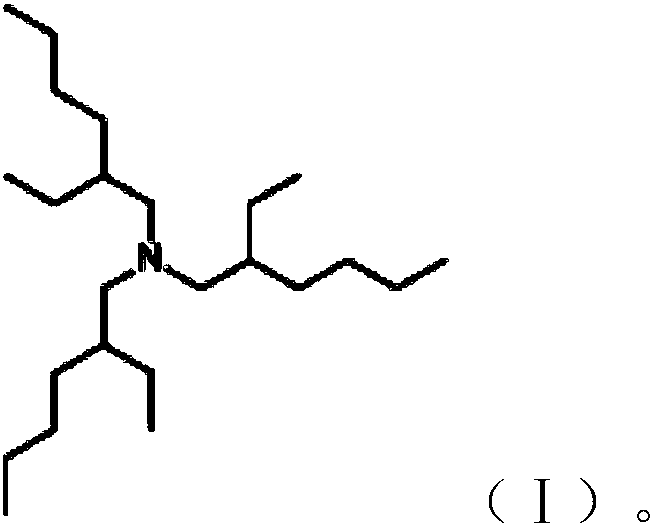
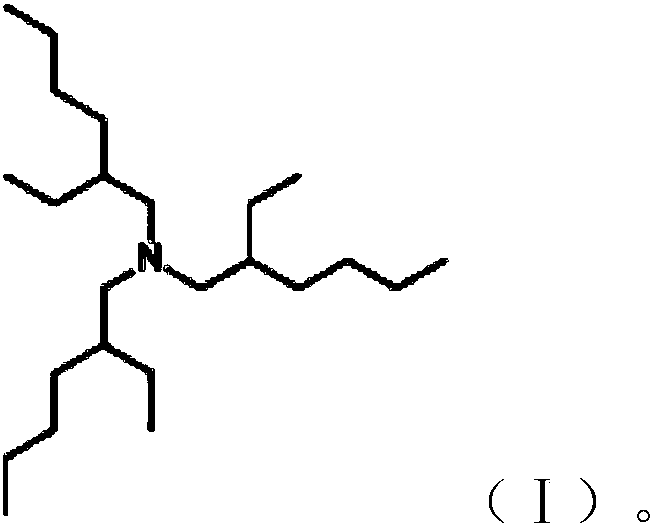
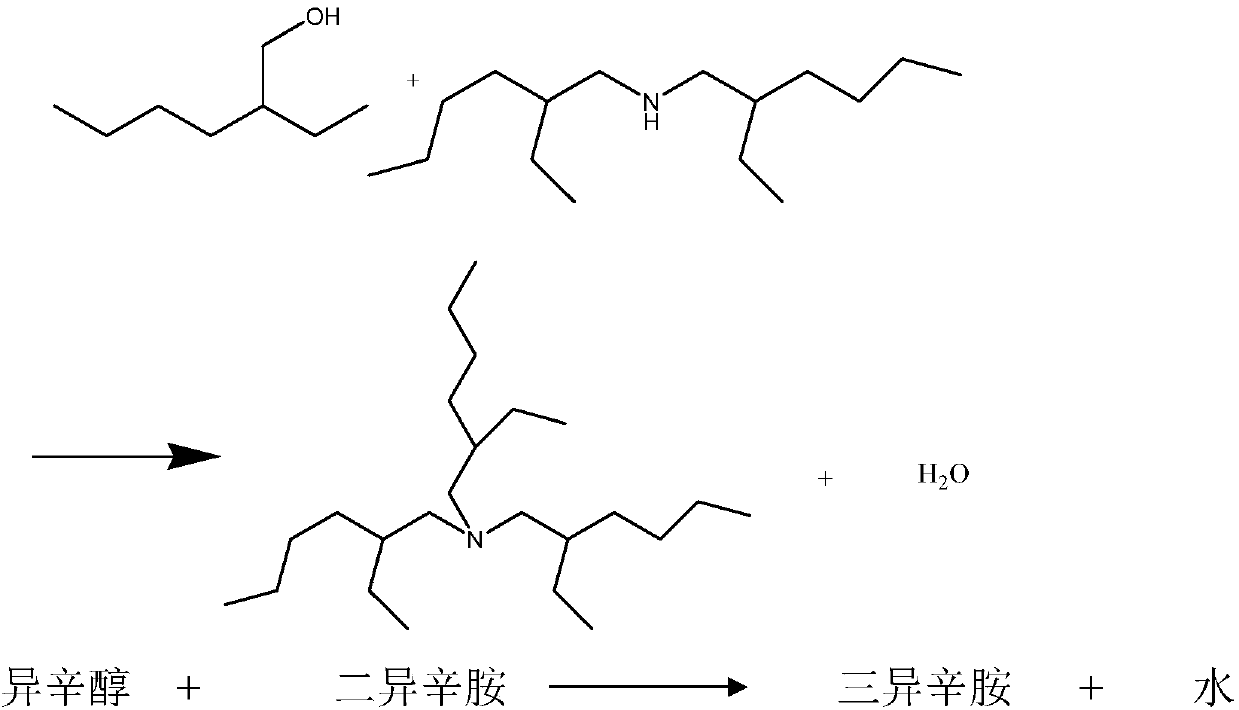
Preparation method of tris(2-ethylhexyl)amine
A technology of triisooctylamine and diisooctylamine, which is applied in the field of preparation of triisooctylamine, can solve the problems of unfavorable industrial production, difficult acquisition of raw material isooctyl aldehyde, and high price, and achieves simple post-processing, simple operation, high cost, and high cost. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Catalyst preparation: Cu(NO 3 )2·3H 2 O, Ni(NO 3 )2·6H 2 O, Zn(NO 3 )2·6H 2 O, Mg(NO 3 )2·6H 2 Dissolve O in a certain amount of water, acetone or ethylene glycol, and then add ZrO corresponding to the volume of the solution 2 As a catalyst carrier, stir evenly to form a paste, age in a water bath at 50±5°C for 5±1h, filter and dry with suction, roast at 450±10°C for 4±0.5h, and crush to below 200 mesh to obtain the catalyst.
[0043] Put diisooctylamine (400g) and catalyst (20g) into a 1-liter four-necked reaction flask equipped with a hydrogen circulation and water separation device. After nitrogen replacement and hydrogen replacement, reduce the catalyst at 200-210°C for 2 hours, then add isooctyl alcohol (260g) dropwise, the reaction temperature is 200-210°C, water is continuously generated during the reaction, and after 20 hours, almost no water is produced. Sampling GC analysis, di-isooctylamine 1%, tri-isooctylamine 74%. The obtained crude product is dir...
Embodiment 2
[0045] Catalyst preparation: Cu(NO 3 )2·3H 2 O, Ni(NO 3 )2·6H 2 O, Zn(NO 3 )2·6H 2 O, Mg(NO 3 )2·6H 2 O is dissolved in a certain amount of water, acetone or ethylene glycol, and then Ca is added corresponding to the volume of the solution 2 CO 3 As a catalyst carrier, stir evenly to form a paste, age in a water bath at 50±5°C for 5±1h, filter and dry with suction, roast at 450±10°C for 4±0.5h, and crush to below 200 mesh to obtain the catalyst.
[0046] Diisooctylamine (450g) and catalyst (20g) were put into a 1 liter four-necked reaction flask equipped with a hydrogen circulation and water separation device. After nitrogen replacement and hydrogen replacement, reduce the catalyst at 190-200°C for 2 hours, then add isooctyl alcohol (260g) dropwise, the reaction temperature is 200-210°C, water is continuously generated during the reaction, and after 18 hours, almost no water comes out. Sampling GC analysis, di-isooctylamine 2%, tri-isooctylamine 80%. The obtained cru...
Embodiment 3
[0048] Catalyst preparation: Cu(NO 3 )2·3H 2 O, Ni(NO 3 )2·6H 2 O, dissolved in a certain amount of water, acetone or ethylene glycol, and then add Ca corresponding to the volume of the solution 2 CO 3 As a catalyst carrier, stir evenly to form a paste, age in a water bath at 50±5°C for 5±1h, filter and dry with suction, roast at 450±10°C for 4±0.5h, and crush to below 200 mesh to obtain the catalyst.
[0049] Diisooctylamine (450g) and catalyst (20g) were put into a 1 liter four-necked reaction flask equipped with a hydrogen circulation and water separation device. After nitrogen replacement and hydrogen replacement, reduce the catalyst at 190-200°C for 2 hours, then add isooctyl alcohol (260g) dropwise, the reaction temperature is 200-210°C, water is continuously generated during the reaction, and after 18 hours, almost no water comes out. Sampling GC analysis, di-isooctylamine 3%, tri-isooctylamine 78%. The obtained crude product is directly used in the rectification ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com