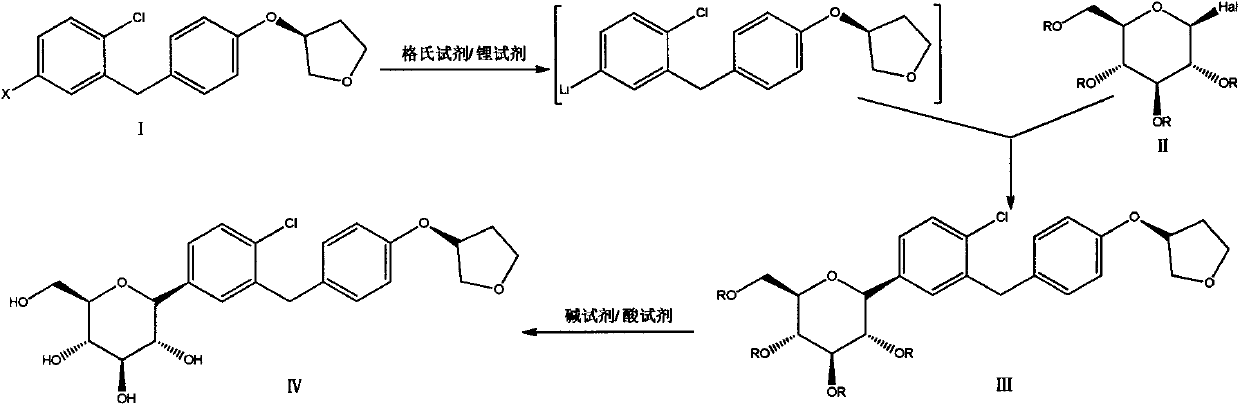
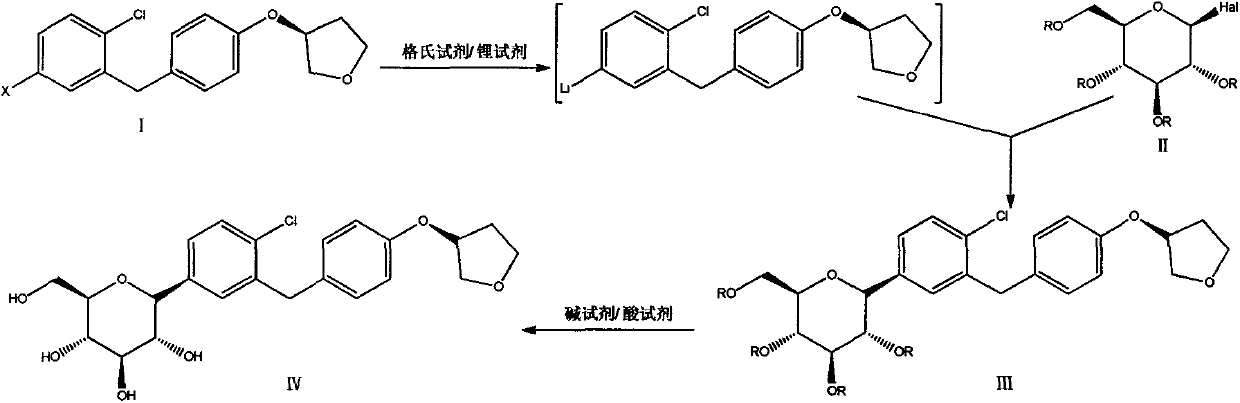
A method for preparing empagliflozin
A technology of empagliflozin and Grignard reagents, which is applied in the field of preparing empagliflozin compounds, can solve the problems of easy isomerization of products, many operation steps, and low reaction temperature, and achieve the reduction of product impurity content and high product purity , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under nitrogen protection, dry THF (120 mL) was added to a 500 mL three-necked flask, cooled to -10 ° C, and a THF solution of i-PrMgCl LiCl (27.2 mL, 27.2 mmol) and n-BuLi in n-hexane ( 21.8mL, 54.4mmol), stirred for 30min. (S)-4-Bromo-1-chloro-2-(4-tetrahydrofuran-3-yloxy-benzyl)benzene (10.0 g, 27.2 mmol) was dissolved in dry THF (10 mL) and slowly added dropwise to In a three-necked flask, the reaction was stirred at -10°C for 2.0 h. Keeping at -10°C, a THF (10 mL) solution of 2,3,4,6-tetraacetoxy-α-D-glucopyranose bromide (11.2 g, 27.2 mmol) was slowly added dropwise, and reacted for 3.0 h. After the reaction was completed, a solution of 6N HCl (20 mL) in methanol (100 mL) was added, the temperature was raised to 25 ° C, stirred for 20.0 h, and saturated NaHCO 3 The pH of the solution was adjusted to 7, extracted twice with EA (100mL), the organic phase was washed with water (100mL) and saturated NaCl solution (100mL) successively, and anhydrous NaCl was added 2...
Embodiment 2
[0037] Under nitrogen protection, add dry THF (40 mL) and 40 mL of toluene to a 500 mL three-necked flask, cool to -10 °C, add n-BuMgCl in THF (12.1 mL, 12.1 mmol) and n-BuLi in n-hexane (9.7mL, 24.2mL), stirred for 20min. Dissolve (S)-4-iodo-1-chloro-2-(4-tetrahydrofuran-3-yloxy-benzyl)benzene (5.0 g, 12.1 mmol) in dry toluene (20 mL) and slowly add dropwise to In the reaction liquid, react at -10°C for 1.0 h. Keeping at -10°C, a solution of 2,3,4,6-tetraacetoxy-α-D-glucopyranose bromide (5.0 g, 12.1 mmol) in toluene (20 mL) was slowly added dropwise and reacted for 2.0 h. After the reaction was complete, a solution of methanesulfonic acid (5.6 mL) in methanol (50 mL) was added, and the temperature was slowly raised to 25° C., stirred for 16.0 h, and saturated NaHCO 3 The pH of the solution was adjusted to 7, extracted twice with EA (100mL), the organic phase was washed with water (100mL) and saturated NaCl solution (100mL) successively, washed with anhydrous NaCl 2 SO 4 ...
Embodiment 3
[0040] Under nitrogen protection, add dry THF (40mL) and toluene (40mL) into a 500mL three-necked flask, cool to -10°C, add a THF solution of n-BuMgCl LiCl (27.2mL, 27.2mmol) and n-BuLi n-hexane solution (21.8mL, 54.4mL), stirred for 10min. Dissolve (S)-4-bromo-1-chloro-2-(4-tetrahydrofuran-3-yloxy-benzyl)benzene (10.0 g, 27.2 mmol) in dry toluene (20 mL) and add dropwise to the reaction solution, reacted at -10°C for 1.0h. Then a solution of 2,3,4,6-tetraacetoxy-α-D-glucopyranose bromide (11.2 g, 27.2 mmol) in toluene (20 mL) was slowly added dropwise, and the reaction was maintained at -10°C for 3.0 h. After the reaction was completed, a solution of methanesulfonic acid (5.2 mL) in methanol (80 mL) was added, and the temperature was slowly raised to 30° C., stirred for 20 h, washed with saturated NaHCO 3 The pH of the solution was adjusted to neutral, extracted twice with EA (100mL), the organic phase was washed with water (100mL) and saturated NaCl solution (100mL) succes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com