A triplet photosensitizer, its preparation method and an upconversion system
A photosensitizer, triplet technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of low up-conversion luminous efficiency, difficult to achieve natural visible light applications, etc., and achieve the effect of strong absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention also provides a kind of preparation method of above-mentioned photosensitizer, comprises the following steps:
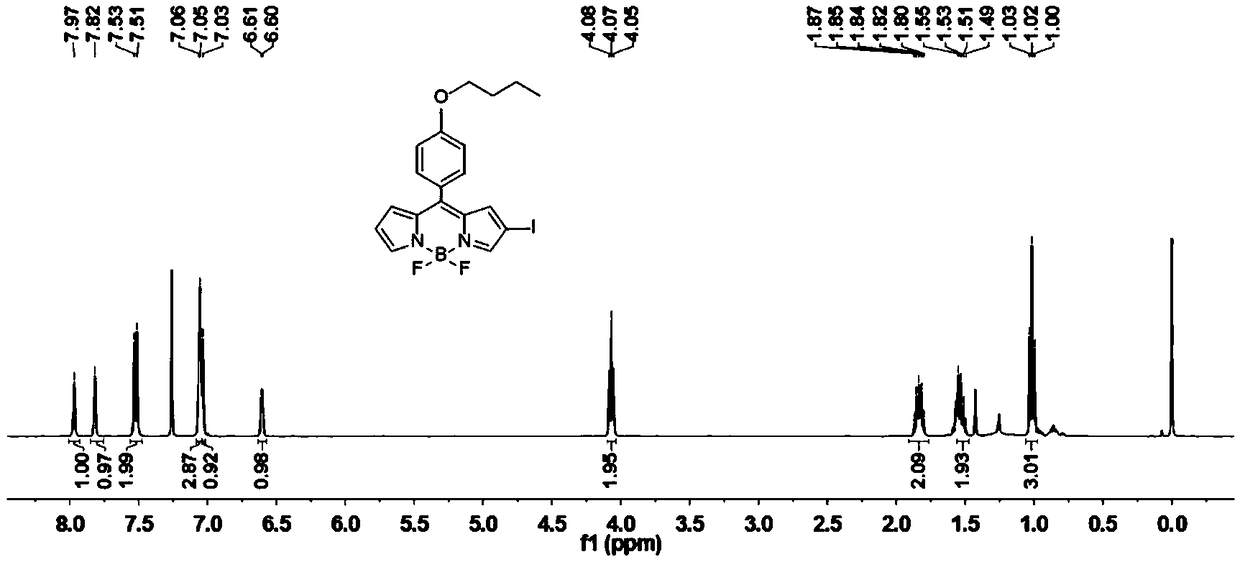
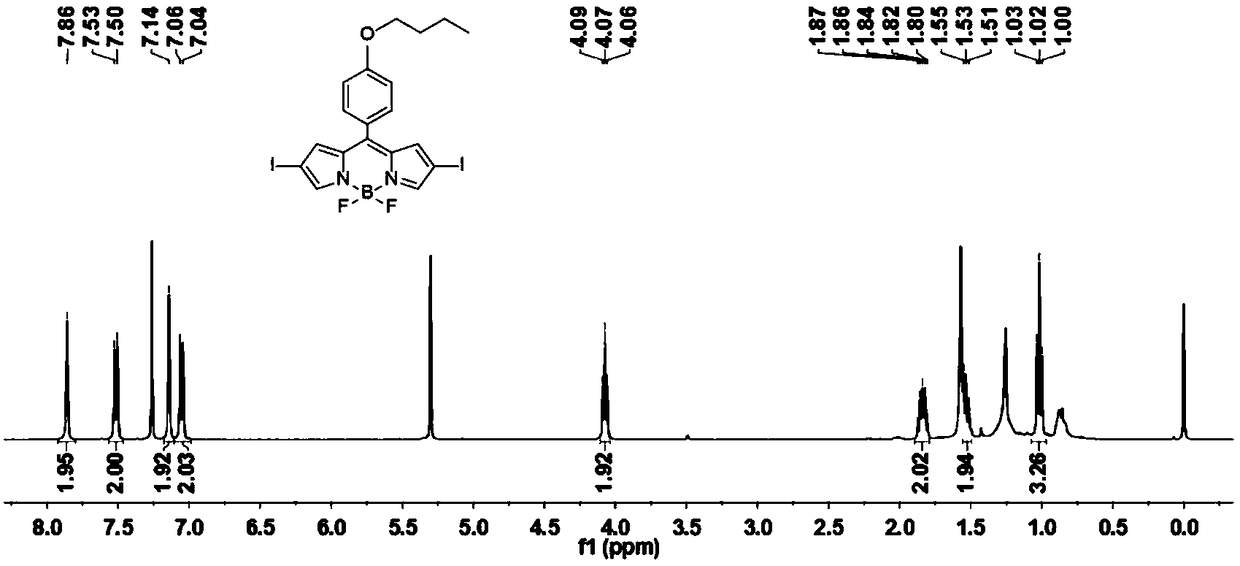
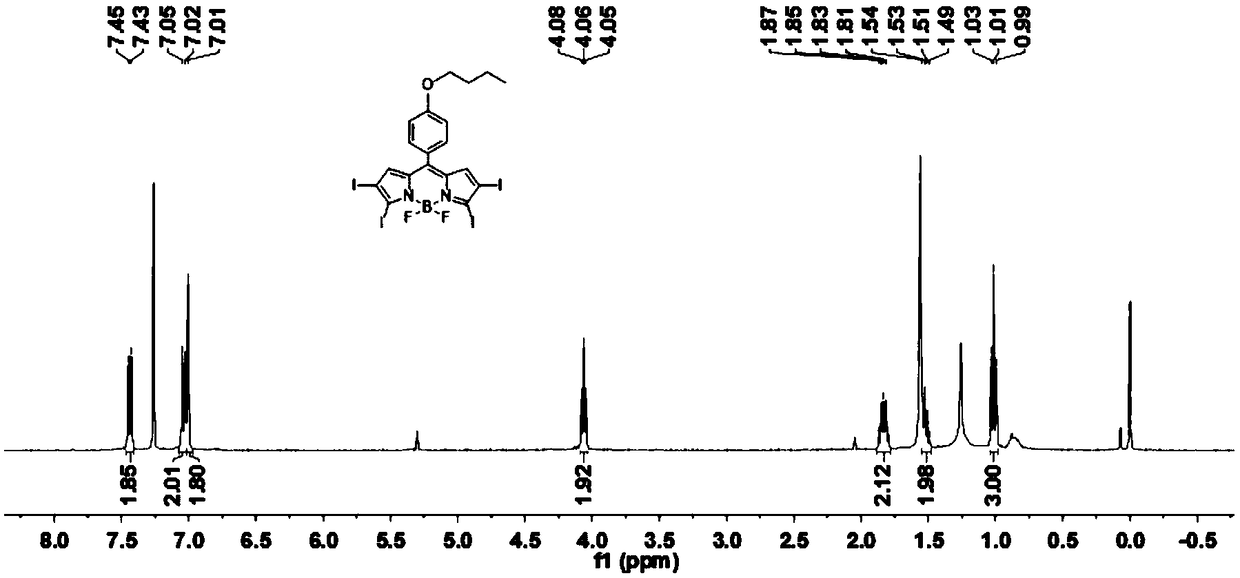
[0034] Have the compound of structure shown in formula (I) and I 2 and HIO 3 An iodination reaction occurs to obtain a photosensitizer having a structure shown in formula (BDP-1) to formula (BDP-3);
[0035]
[0036] Specifically, under protective atmosphere conditions, the compound having the structure shown in formula (I), elemental iodine and a saturated solution of iodic acid are mixed and heated for reaction to obtain a reaction product;
[0037] The reaction product is purified to obtain photosensitizers having structures represented by formula (BDP-1) to formula (BDP-3).
[0038] Wherein, the BDP-1 protective atmosphere condition is preferably nitrogen, and the molar ratio of the compound having the structure represented by formula (I) to iodine is 1:5-1:10. The reaction temperature is 30-40 degrees Celsius, and the reaction ...
Embodiment 1
[0077] Prepare the compound with structure shown in formula (II) by p-hydroxybenzaldehyde and n-bromobutane
[0078] P-Hydroxybenzaldehyde (2.44g, 20mmol), potassium carbonate (4.15g, 30mmol) were dissolved in 50ml of DMF solution. After heating to reflux for 1 h, the temperature was lowered to 35° C., n-bromobutane (20 mmol, 2.72 g) was slowly added, and after stirring for 10 min, the temperature was raised to reflux for 12 h. After cooling to room temperature, the solvent was removed by rotary evaporation, extracted with dichloromethane and water, dried over anhydrous sodium sulfate, the solvent was removed by rotary evaporation, and purified by petroleum ether and ethyl acetate (volume ratio: 1:1). 3.04 g of yellow oily liquid, that is, the compound having the structure shown in formula (II). The yield was determined to be 85.4%.
[0079]
[0080] NMR data: 1 H NMR (400MHz, CDCl 3 )δ9.88(s,1H),7.82(d,J=8.0Hz,2H),6.99(d,J=8.0Hz,2H),4.05(s,2H),1.88–1.73(m,2H), 1.52(t,...
Embodiment 2
[0082] Prepare the compound (BDP) with the structure shown in the formula (I) by the compound with the structure shown in the formula (II) and pyrrole
[0083] A compound (8.43mmol, 1.5g) having a structure shown in formula (II) was dissolved in 150ml of dry dichloromethane, and under nitrogen protection, pyrrole (1.13g, 16.86mmol, 1.18ml) and 0.15ml of trifluoro Acetic acid catalyst, at room temperature (after adding trifluoroacetic acid, the color gradually deepens, and after half an hour, the solution turns black.) After reacting for 10 hours, add DDQ (8.43mmol, 1.91g), react for 1 hour, and then cool with ice water, Add triethylamine (10equiv, 11.6ml), react for 30min, and then add boron trifluoride ether (11.6ml). The reaction was stopped after 30 min of reaction. Remove the solvent by rotary evaporation, add a large amount of water to remove triethylamine and boron trifluoride ether, then add dichloromethane and water to extract, dry with anhydrous sodium sulfate, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com