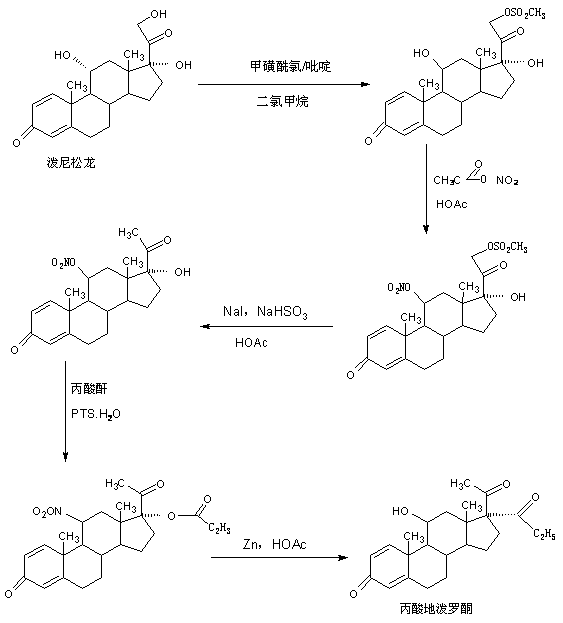
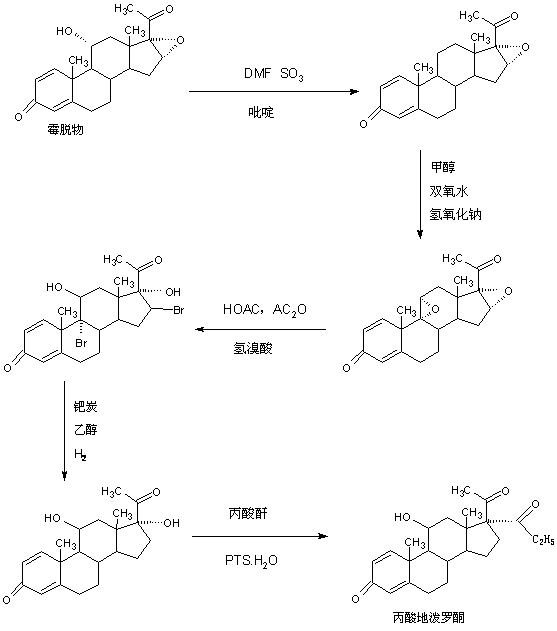
Preparation method of deprodone propionate
A technology of dipronone propionate and dipronone propionate, which is applied in the field of preparation of local anti-inflammatory hormone drug dipronone propionate, can solve the problems of low synthesis efficiency and high production cost, and achieve low production cost and high product quality. Good quality, simple process operation and environmental protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A. Preparation of dehydrated matter
[0040] In a 2000ml three-necked flask, add 100g of mildew and 1200ml of pyridine, stir to dissolve completely, then pass in sulfur trioxide at 30-35°C, keep the pressure at about 1.5atm, ventilate for 10 to 12 hours, confirm by TLC At the end of the reaction, after the reaction, remove sulfur trioxide in vacuum, then add 100ml of 30% caustic soda solution and 100ml of saturated saline to wash once, then concentrate under reduced pressure to recover 90-95% of pyridine, and finally add 600ml of tap water, Stir and crystallize at 10-15°C for 3-4 hours, centrifuge, wash with water to obtain the crude dehydrated product, add the above-mentioned crude product to 500ml 20% ethanol aqueous solution directly without drying, stir and crystallize at 0-5°C for 5-6 hours, centrifuge, Washed with water and dried to obtain 85 g of dehydrated product, the HPLC content was 99.2%, and the weight yield was 85%.
[0041] B. Preparation of epoxy
[00...
Embodiment 2
[0050] A. Preparation of dehydrated matter
[0051] In a 2000ml three-necked bottle, add 100g of mildew and 1200ml of chloroform, stir to dissolve completely, then add 120ml of pyridine, then pass in sulfur trioxide at 30-35°C, keep the pressure at about 1.5atm, and ventilate for 10~ After 12 hours, TLC confirmed the end point of the reaction. After the reaction, remove sulfur trioxide in vacuum, then add 100ml of 30% caustic soda solution and 100ml of saturated saline to wash once, then concentrate under reduced pressure to recover 90-95% of chloroform and pyridine , finally add 600ml of tap water, stir and crystallize at 10-15°C for 3-4 hours, centrifuge, wash with water to get the crude dehydrated product, directly add the above crude product to 500ml of 20% ethanol aqueous solution without drying, and stir at 0-5°C Crystallize for 5-6 hours, centrifuge, wash with water, and dry to obtain 84.5 g of dehydrated product, with an HPLC content of 99.3% and a weight yield of 84.5...
Embodiment 3
[0061] A. Preparation of dehydrated matter
[0062]In a 2000ml three-necked bottle, add 100g of mildew and 1200ml of toluene, stir to dissolve completely, then add 120ml of triethylamine, and then inject sulfur trioxide at 30-35°C, keep the pressure at about 1.5atm, and ventilate the reaction After 10-12 hours, TLC confirms the end point of the reaction. After the reaction, remove sulfur trioxide in vacuum, then add 100ml of 30% caustic soda solution and 100ml of saturated saline to wash once, then concentrate under reduced pressure to recover 90-95% toluene , finally add 600ml of tap water, stir and crystallize at 10-15°C for 3-4 hours, centrifuge, wash with water to get the crude dehydrated product, add the above crude product directly to 500ml of 20% ethanol aqueous solution without drying, stir and crystallize at 0-5°C After 5-6 hours, centrifuge, wash with water, and dry to obtain 84.2 g of dehydrated product with an HPLC content of 99.3% and a weight yield of 84.2%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com