Method for preparing P(VDF-DB)-g-S-C3H6-SO3H proton exchange membrane material
A proton exchange membrane and VDF-DB technology, which is applied in the field of preparing fluoropolymer-based proton exchange membrane materials, can solve the problems of complex preparation process and complex process, and achieve the effect of simple process and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This embodiment includes the following steps:
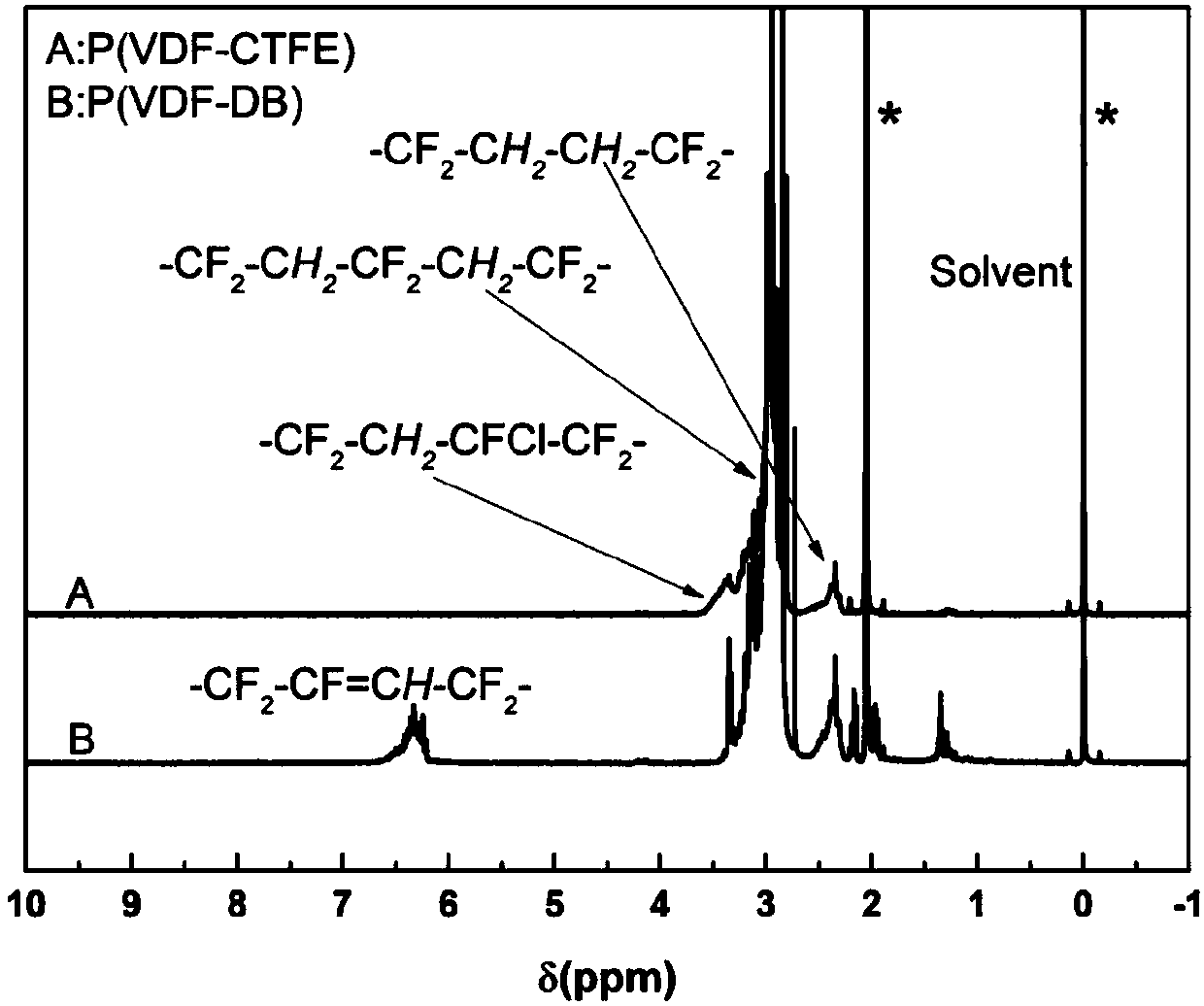
[0027] Step 1: Add the raw material P(VDF-CTFE) into the reaction bottle, and add the solvent N-methylpyrrolidone at the same time. After fully dissolving, add a sufficient amount of catalyst triethylamine, and stir the reaction at 55°C-65°C for 24 hours. The resulting polymer solution P(VDF-DB) was precipitated with deionized water, while excess triethylamine was removed with hydrochloric acid. After soaking the precipitated polymer in methanol, it is vacuum-dried to constant weight at no higher than 50°C. At this time, the structure can be characterized by nuclear magnetic resonance. The hydrogen nuclear magnetic resonance spectrum can be seen in figure 1 , it can be clearly seen that P(VDF-DB) has a new multiple peak at 6.1-6.5ppm than the raw material, and this peak corresponds to the chemical shift of (-CF2-CF=CH-CF2-)H on the double bond .
[0028] Step two:
[0029] Add the polymer P(VDF-DB) obtained in step 1 in...
Embodiment 2
[0037] This embodiment includes the following steps:
[0038] Step 1: Add the raw material P(VDF-CTFE) into the reaction bottle, and add the solvent at the same time. After fully dissolving, add a sufficient amount of catalyst triethylamine, and stir the reaction at 55°C-65°C for 24 hours. The resulting polymer solution P(VDF-DB) was precipitated with deionized water, while excess triethylamine was removed with hydrochloric acid. The precipitated polymer is soaked in methanol, and vacuum-dried to a constant weight at a temperature not higher than 50°C.
[0039] Step two:
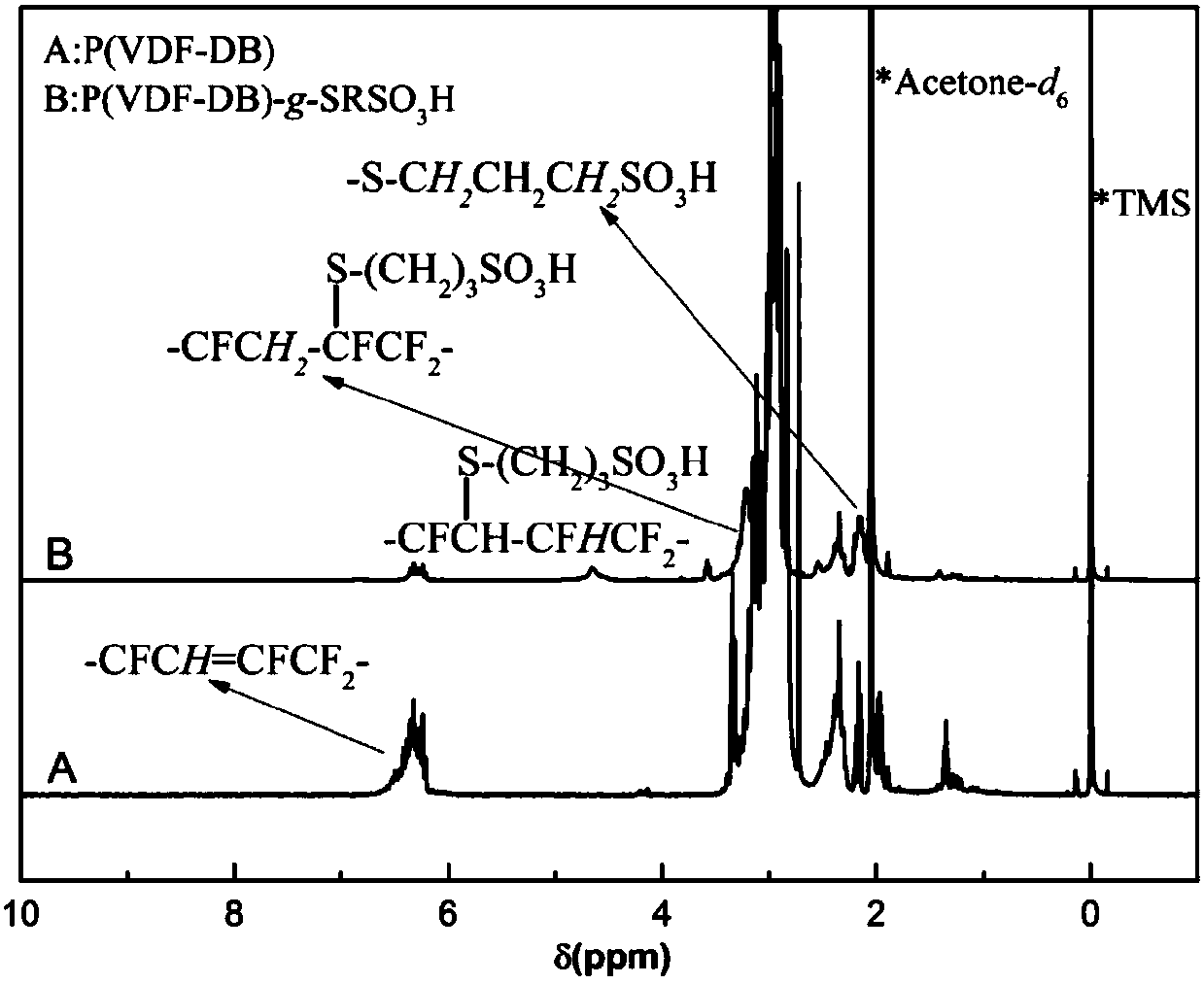
[0040] Add the polymer P(VDF-DB) obtained in step 1 into the reaction flask, the chemical composition of P(VDF-DB) is VDF / DB=86:14, add a solvent to fully dissolve. Then add an appropriate amount of 3-mercapto-1-propanesulfonate sodium and an appropriate amount of deionized water. After the sodium mercaptosulfonate is completely dissolved, add an appropriate amount of catalyst in the reaction solution, whe...
Embodiment 3
[0047] This embodiment includes the following steps:
[0048] Step 1: Add the raw material P(VDF-CTFE) into the reaction bottle, and add the solvent at the same time. After fully dissolving, add a sufficient amount of catalyst triethylamine, and stir the reaction at 55°C-65°C for 24 hours. The resulting polymer solution P(VDF-DB) was precipitated with deionized water, while excess triethylamine was removed with hydrochloric acid. The precipitated polymer is soaked in methanol, and vacuum-dried to a constant weight at a temperature not higher than 50°C.
[0049] Step two:
[0050] Add the polymer P(VDF-DB) obtained in step 1 into the reaction flask, the chemical composition of P(VDF-DB) is VDF / DB=86:14, add a solvent to fully dissolve. Then add an appropriate amount of 3-mercapto-1-propanesulfonate sodium and an appropriate amount of deionized water. After the sodium mercaptosulfonate is completely dissolved, add an appropriate amount of catalyst in the reaction solution, whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com