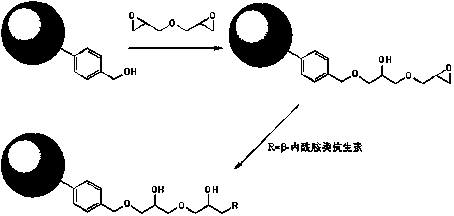
Beta-lactan antibiotic grafted polymer and application thereof
A technology of grafting polymers and lactams, applied in the field of biochemical analysis, can solve the problems of heavy workload, long time, inability to generate new lactamase genes and mutant genes, etc., and achieve the effect of reducing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Cefepime Grafted Polymer
[0024] Step 1: Add 100ml of dichloroethane into the three-necked flask, add 10g of hydroxymethyl polystyrene resin and 20g of bisglycidyl ether into the reactor and mix evenly, slowly add 1ml of 40% sodium hydroxide aqueous solution dropwise at room temperature, After reacting for 5 hours, filter, and the filter cake is fully washed with 200ml ethylene dichloride to obtain a grafted polymer;
[0025] Step 2: Disperse the grafted polymer obtained by the product of step 1) in 100ml THF solution, add 20g cefepime into the reactor to carry out ring-opening reaction, react for 8 hours, filter the reaction solution, and filter the filter cake fully with 200ml THF Washing to finally obtain the cefepime grafted polymer;
[0026] Step 3: Suspend the cefepime grafted polymer obtained in step 2) in 50mM Tris, PBS of pH 7.0, adopt a wet packing method, and fill 1 ml of gel into each column. After the column is installed, use 5ml , 0.01%Na...
Embodiment 2
[0028] Preparation of Ceftaroline Grafted Polymer
[0029] Step 1: Add 100ml tetrahydrofuran into the three-necked flask, add 10g hydroxymethyl polystyrene resin and 20g bisglycidyl ether into the reactor and mix evenly, slowly add 0.3g SDS at room temperature, react for 5 hours, filter, filter cake Fully wash with 200ml tetrahydrofuran to obtain graft polymer;
[0030] Step 2: Disperse the graft polymer obtained by the product of step 1) in 100ml DMF solution, add 20g cefepime into the reactor to carry out ring-opening reaction, react for 8 hours, filter the reaction solution, and wash the filter cake fully with 200ml DMF , to finally obtain the cefepime grafted polymer;
[0031] Step 3: Suspend the cefepime grafted polymer obtained in step 2) in 50mM Tris, PBS of pH 7.0, adopt a wet packing method, and fill 1 ml of gel into each column. After the column is installed, use 5ml , 0.01%NaN 3 -PBS sterile buffer continues to pass through the column. When the top of the column ...
Embodiment 3
[0033] Preparation of Cefditoren Axetil Graft Polymer
[0034] Step 1: Add 100ml of DMF to the three-necked flask, add 10g of hydroxymethyl polystyrene resin and 20g of bisglycidyl ether into the reactor and mix evenly, slowly add 0.4g of 16-alkyl sodium sulfate at room temperature, and react for 5 hours , filter, and the filter cake is fully washed with 200ml DMF to obtain a grafted polymer;
[0035] Step 2: Disperse the graft polymer obtained by the product of step 1) in 100ml of dichloromethane solution, add 20g of cefepime into the reactor to carry out ring-opening reaction, react for 8 hours, filter the reaction solution, and use 200ml Dichloromethane fully washes, finally obtains cefditoren axetil graft polymer;
[0036] Step 3: The cefditoren axetil graft polymer obtained in step 2) was suspended in 50 mM Tris, pH 7.0 PBS. Wet column packing is adopted, each column is loaded with 1 ml gel, after the column is installed, use 5ml, 0.01% NaN 3 -PBS sterile buffer contin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com