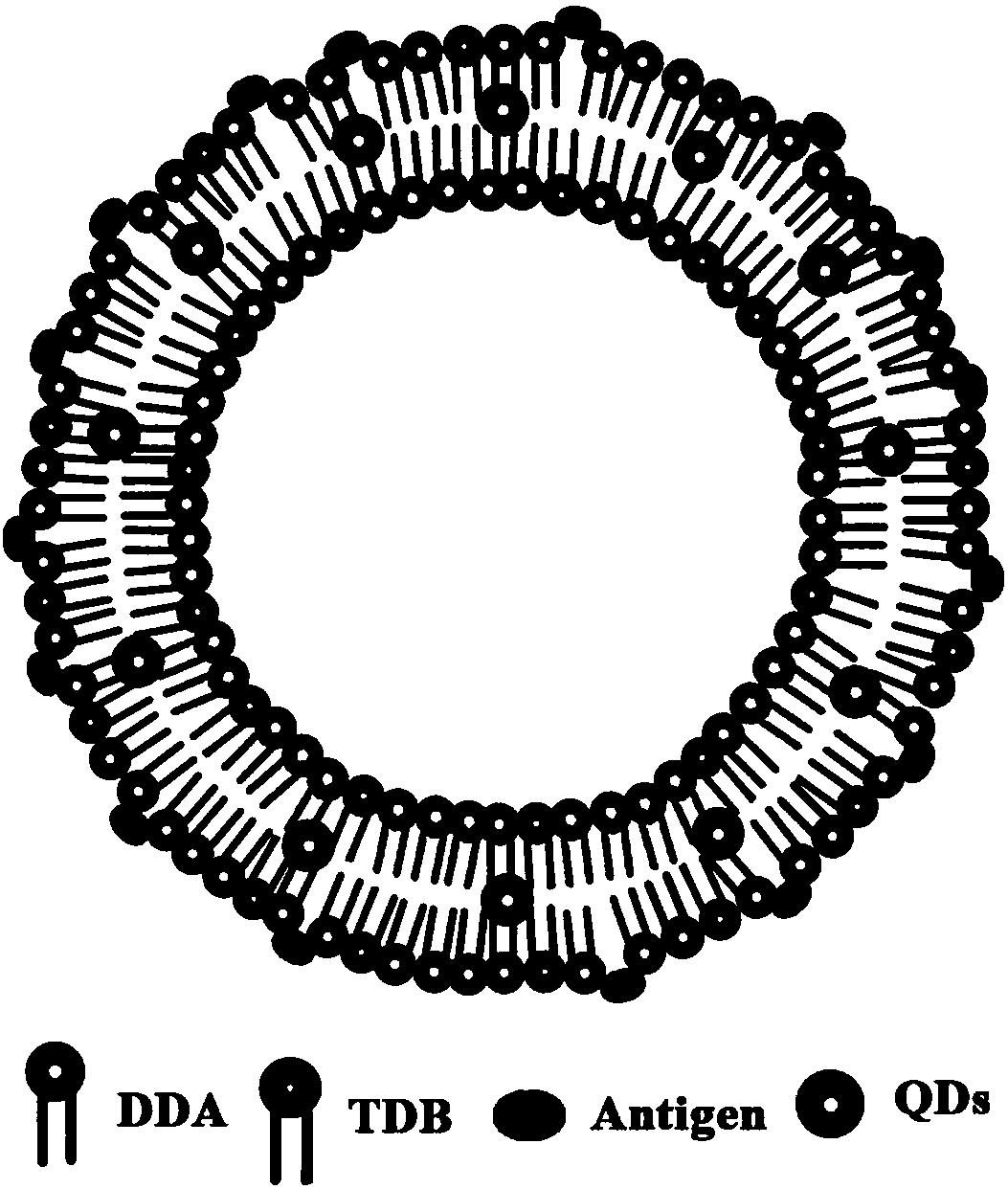
Cationic liposome influenza vaccine capable of entrapping quantum dots and preparation method thereof
A cationic liposome and influenza vaccine technology, which is applied in the field of novel particle drug delivery system, cationic liposome influenza vaccine and its preparation, can solve the problems of poor patient compliance, single route of vaccination, weak immunogenicity, etc. Toxic side effects, improved fluorescence stability, high luminous intensity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: film dispersion method prepares DDA-TDB liposome influenza vaccine
[0055] Put DDA and TDB in a 50 ml round bottom flask with a molar ratio of 7 to 10:1, add 1 to 2 ml of chloroform and methanol to dissolve, add quantum dots, and remove the organic solvent by rotary evaporation under reduced pressure under heating in a water bath to form a uniform Lipid film, and then pass through 3minN 2 , remove the residual solvent. Then 2 ml of 10 mM Tris-HCl buffer (pH 6.8-9) was added, ultrasonicated in a water bath at 60°C and incubated for 45 min to obtain blank liposomes with a particle size of about 600-700 nm. Then, according to the influenza vaccine: DDA-TDB mass ratio of 10-12.5:100, the influenza vaccine stock solution was added to the above-mentioned blank liposomes to make them fully fused, and stored at 4°C for future use. The particle size of the cationic liposome influenza vaccine obtained above is 1700-1900nm, and compared with the blank liposome, th...
Embodiment 2
[0056] Embodiment 2: film dispersion method prepares DOTAP-DC-Chol liposome influenza vaccine
[0057] Put DOTAP and DC-Chol in a 1:1 molar ratio in a 50 ml round bottom flask, add 1-2 ml
[0058] Dissolve chloroform and methanol, add quantum dots, and remove the organic solvent by rotary evaporation under reduced pressure under water bath heating to form a uniform lipid film, and then pass through 3minN 2 , remove the residual solvent. Then add 2 ml of 10 mM Tris-HCl buffer solution (pH 6.8-7.4), sonicate in a water bath at 60°C and incubate for 30 min to obtain blank liposomes with a particle size of about 250-270 nm. Then, according to the mass ratio of influenza vaccine:DOTAP-DC-Chol of 5-12.5:100, the stock solution of influenza vaccine was added to the above-mentioned blank liposomes to make them fully fused, and stored at 4°C for future use. The cationic liposome influenza vaccine obtained above has a particle size of 330-350nm, a zeta of 49-54mV, an encapsulation eff...
Embodiment 3
[0059] Embodiment 3: film dispersion method prepares DOTAP-DC-Chol liposome influenza vaccine
[0060] Put DOTAP and DC-Chol in a 1:1 molar ratio in a 50 ml round bottom flask, add 1-2 ml
[0061] Dissolve in chloroform and methanol, and remove the organic solvent by rotary evaporation under reduced pressure under heating in a water bath at 35-40°C to form a uniform lipid film, and then pass it through for 3minN 2 , remove the residual solvent. Then add 2 ml of 10 mM Tris-HCl buffer (pH 6.8-7.4), sonicate in a water bath at 55-65°C and incubate for 30 min to obtain blank liposomes with a particle size of about 230-290 nm. Then, according to the mass ratio of influenza vaccine: DOTAP-DC-Chol of 5-12.5:100, the stock solution of influenza vaccine was added to the above blank liposome, and quantum dots were added to make it fully fused, and stored at 4°C for future use. The cationic liposome influenza vaccine obtained above has a particle size of 310-370nm, a zeta of 45-57mV, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com