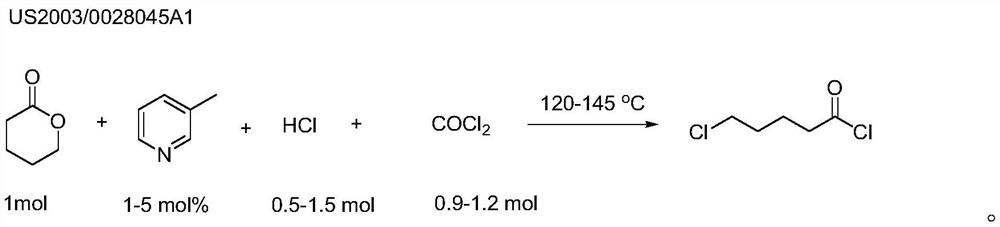
A kind of preparation method of 5-chloropentanoyl chloride
A technology of chlorovaleryl chloride and valerolactone is applied in the preparation of acyl halide, organic chemistry and other directions, which can solve the problems of large pollution and troublesome handling, and achieve the effects of high synthesis efficiency, simplified operation and high catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a two-necked round-bottomed flask (one is connected to the condenser and the other is used as passing through phosgene), add δ-valerolactone (100g, 1mol) successively, catalyst Fe powder (0.5g, 0.5wt%) and water ( 2g, 2wt%), heated the mixed solution to 100°C and started to feed phosgene (107g, 1.1mol). The time for feeding phosgene was about 4 hours, during which the temperature was maintained at about 100°C. After adding phosgene, continue to react at about 80°C for 1 hour, then cool down the reaction solution to room temperature, and replace excess phosgene with nitrogen. The reaction solution was distilled under reduced pressure and the fraction at 120°C (1.0kPa) was collected to obtain 5-chlorovaleryl chloride (142g, yield 93%).
[0030] Replacement example:
[0031] The preparation method is the same as in Example 1, the difference is that the amount of Fe catalyst, water, phosgene, and reaction temperature are adjusted, and the impact on the reaction yield is...
Embodiment 2
[0035] In a two-necked round-bottomed flask (one is connected to the condenser tube and the other is used as passing through phosgene), add δ-valerolactone (100g, 1mol) successively, and the catalyst Fe 2 o 3 (1g, 1wt%) and water (2g, 2wt%), begin to pass into phosgene (107g, 1.1mol) after the mixed solution is heated up to 100 ℃, the time of passing into phosgene is about 4 hours, and temperature maintains during At around 100°C. After adding phosgene, continue to react at about 80°C for 1 hour, then cool down the reaction solution to room temperature, and replace excess phosgene with nitrogen. The reaction solution was distilled under reduced pressure and the fraction at 120°C (1.0kPa) was collected to obtain 5-chlorovaleryl chloride (122g, yield 80%).
Embodiment 3
[0037] In a two-necked round-bottomed flask (one is connected to the condenser tube, the other is used for passing through phosgene), successively add δ-valerolactone (100g, 1mol), catalyst FeCl 3 (1.0g, 1wt%) and water (2g, 2wt%), begin to pass into phosgene (107g, 1.1mol) after the mixed solution is heated up to 100 ℃, the time of passing into phosgene is about 4 hours, during the temperature Maintained at around 100°C. After adding phosgene, continue to react at about 80°C for 1 hour, then cool down the reaction solution to room temperature, and replace excess phosgene with nitrogen. The reaction solution was distilled under reduced pressure and the fraction at 120°C (1.0kPa) was collected to obtain 5-chlorovaleryl chloride (132g, yield 86%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com