Method for synthesizing 2H-azirine amide
A technology for acrylamide and nitrogen heterocycle, which is applied in the field of synthesizing 2H-azacycloacrylamide derivatives, can solve the problems of low yield, small substrate range, high temperature and the like, and achieves high yield and wide substrate range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The first step, synthetic raw material 3-cyclohexyl-5-aminoisoxazole:
[0023]
[0024] Add sodium acetate (2mmol) and hydroxylamine hydrochloride (2mmol) in 10ml reaction tube, then add 1ml methanol, stir
[0025] Stir for one hour, then add cyclohexylformylacetonitrile (1 mmol) in methanol (1 ml) and stir for 12-24 h. After TLC monitors that the reaction is complete, transfer the reaction solution to a 100ml round-bottomed flask, remove methanol with a vacuum rotary evaporator, add 20ml of water, extract three times with ethyl acetate, each time with 10ml of ethyl acetate, combine the organic layers, and use 20ml Wash with saturated brine, dry the organic layer with anhydrous sodium sulfate, remove ethyl acetate with a vacuum rotary evaporator, and separate the product by column chromatography. The developing solvent is polar petroleum ether / ethyl acetate=4:1, and the product is light yellow Solid, yield 80%.
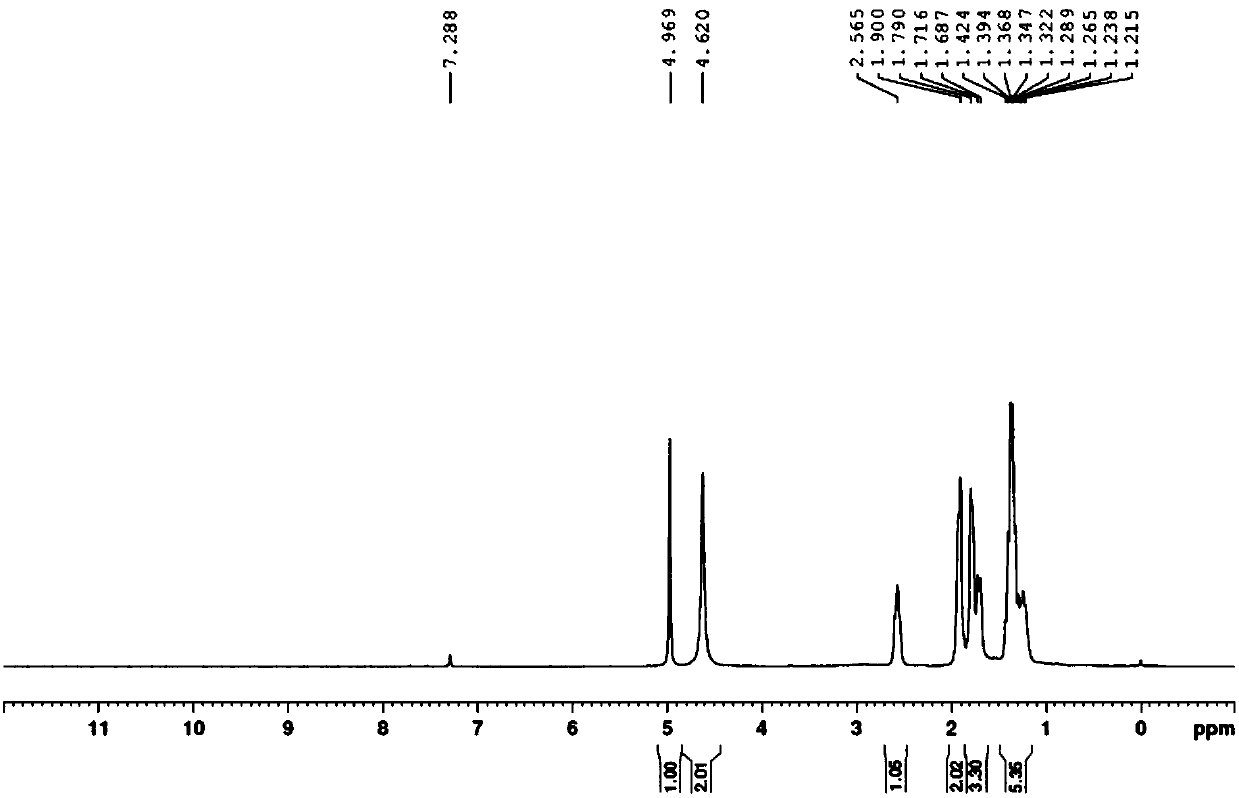
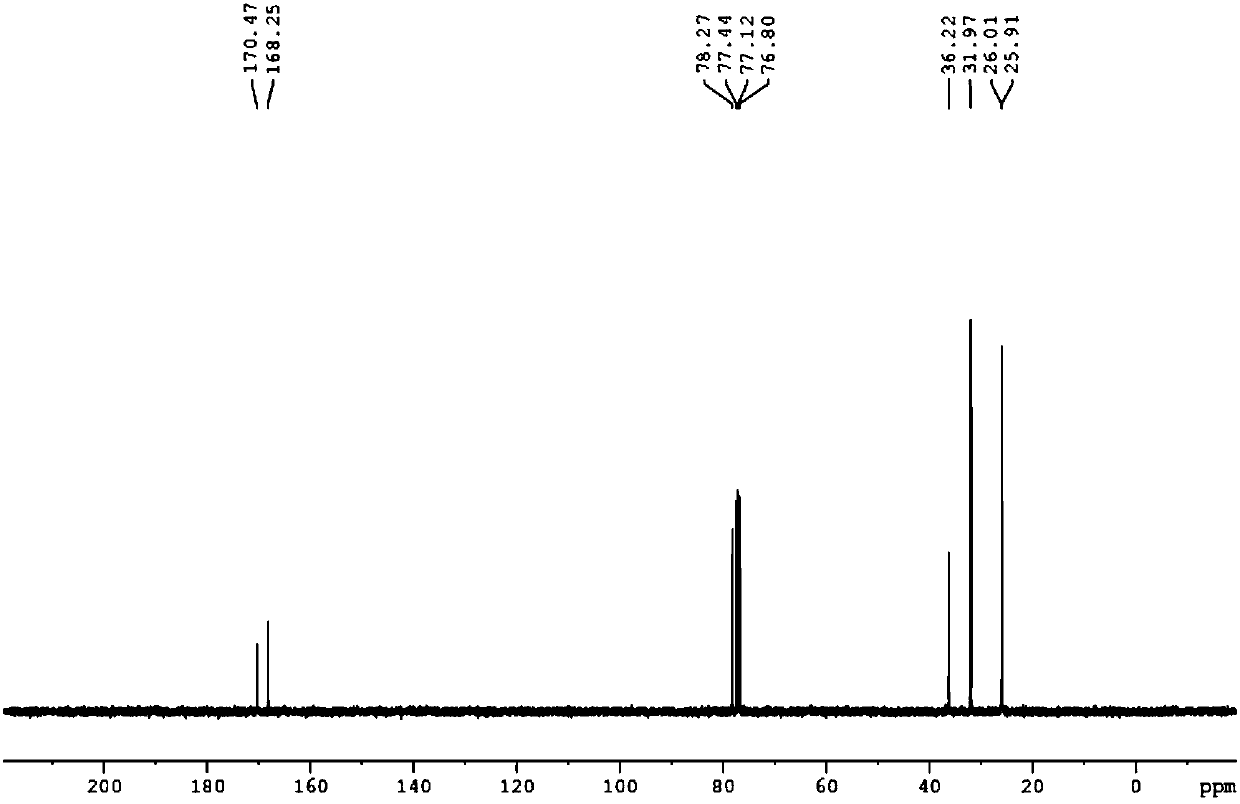
[0026] The H NMR spectrum of 3-cyclohexyl-5-aminoiso...
Embodiment 2
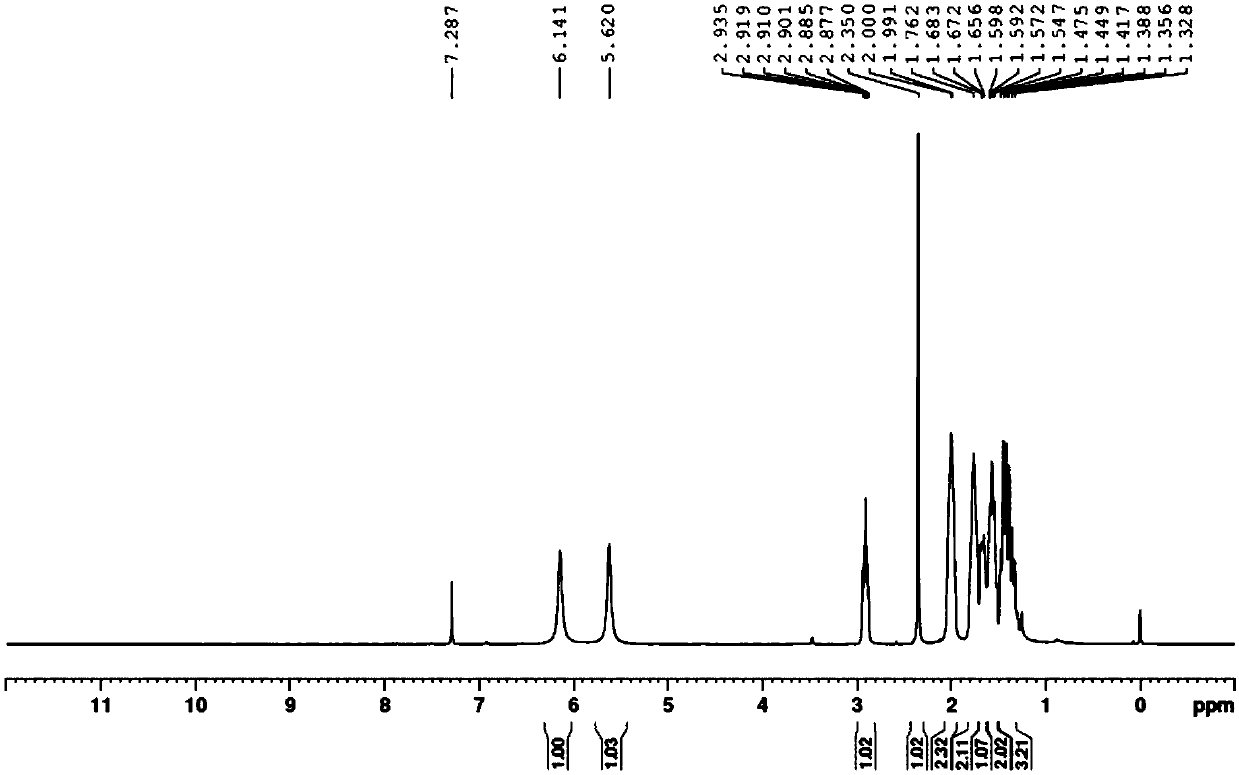
[0032] Add 3-phenylpropyl-5-aminoisoxazole (1mmol) into a 10ml reaction tube, add 2ml of absolute ethanol, add dichloro(o-isopropoxyphenylmethylene)(tricyclohexylphosphine)ruthenium (1mol%), irradiated with 36W green light for 36 hours, after the TLC monitoring reaction was complete, the reaction solution was transferred to a 100ml round-bottomed flask, and the ethanol was removed with a vacuum rotary evaporator, and the product was separated by column chromatography, and the developing agent was two Chloromethane / ethyl acetate=4:1, the product is a white solid, and the yield is 89%.
Embodiment 3
[0034] Add 3-(4-chlorophenyl)isoxazole (1mmol) into a 10ml reaction tube, add 2ml of absolute ethanol, add dichloro[1,3-bis(2-methylphenyl)-2-imidazolidine Subunit] (2-isopropoxybenzylidene) ruthenium (1mol%), irradiated with 36W green light for 32 hours, after the TLC monitoring reaction was complete, the reaction solution was transferred to a 100ml round-bottomed flask, and rotated with a vacuum Ethanol was removed by the evaporator, and the product was separated by column chromatography. The developing solvent was dichloromethane / ethyl acetate=4:1. The product was a white solid with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com