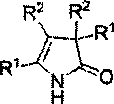
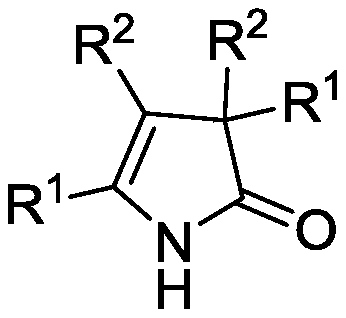
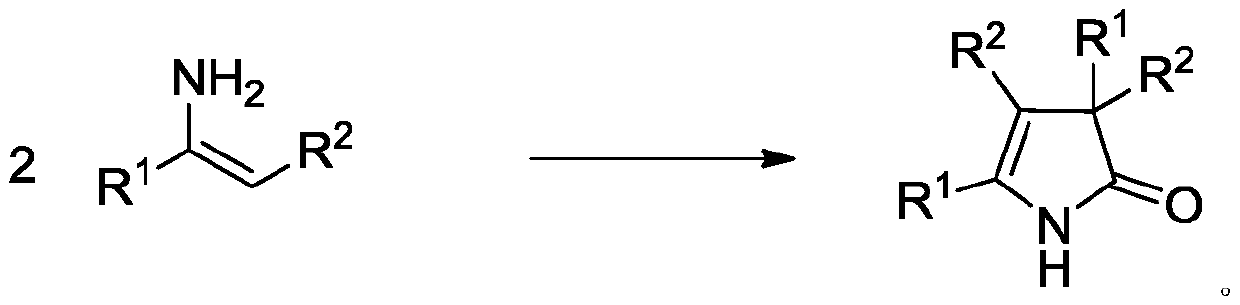
A kind of 3h-2-pyrrolidone compound and its synthetic method
A 3H-2-, pyrrolidone technology, applied in organic chemistry and other fields, can solve the problems of low preparation cost, high synthesis difficulty, and limited synthesis methods of 3H-2-pyrrolidone, and achieve low preparation cost, simple operation, and relative yield and the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: The synthesis of 3H-2-pyrrolidone (2a), its structural formula is specifically as follows:
[0021]
[0022] Add 1mmol of enaminoester (1a) and 1mmol of 2,2,6,6-tetramethylpiperidine-nitroxyl radical into 5mL of ethylene glycol dimethyl ether, then add 1mmol of potassium persulfate, and reflux for 12h. After the reaction was finished, the reaction solution was washed with 10 mL of saturated sodium carbonate solution, then extracted with ethyl acetate (10 mL × 2), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, and the crude product was subjected to fast silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =10:1), the product 155mg was obtained, and the calculated yield was 88%.
[0023] Its main physical and chemical properties are as follows:
[0024] White solid, melting point 144-145°C; 1 H NMR (400MHz, CDCl 3 ):δ8.01(br,1H),7.65-7.68(m,2H),7.56-7.59(m,2H),7.47-7.53(m,3H),7.34-7.39(m,3H),3.74(s ,3H),3.58(s,3H); ...
Embodiment 2
[0025] Embodiment 2: The synthesis of 3H-2-pyrrolidone (2b), its structural formula is specifically as follows:
[0026]
[0027] Add 1mmol of enaminoester (1b) and 1mmol of 2,2,6,6-tetramethylpiperidine-nitroxyl radical into 5mL of ethylene glycol dimethyl ether, then add 1mmol of potassium persulfate, and reflux for 12h. After the reaction was finished, the reaction solution was washed with 10 mL of saturated sodium carbonate solution, then extracted with ethyl acetate (10 mL × 2), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, and the crude product was subjected to fast silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =10:1), the product 170mg was obtained, and the calculated yield was 90%.
[0028] Its main physical and chemical properties are as follows:
[0029] yellow oil; 1 H NMR (400MHz, CDCl 3 ):δ8.61(br,1H),7.64-7.67(m,2H),7.57-7.59(m,2H),7.43-7.50(m,3H),7.33-7.37(m,3H),4.19-4.31 (m,2H),4.03-4.09(m,2H),1.24(t,J=7.1Hz,3H...
Embodiment 3
[0030] Embodiment 3: Synthesis of 3H-2-pyrrolidone (2c), its structural formula is as follows:
[0031]
[0032] Add 1mmol of enaminoester (1c) and 1mmol of 2,2,6,6-tetramethylpiperidine-nitroxyl radical into 5mL of ethylene glycol dimethyl ether, then add 1mmol of potassium persulfate, and reflux for 12h. After the reaction was finished, the reaction solution was washed with 10 mL of saturated sodium carbonate solution, then extracted with ethyl acetate (10 mL × 2), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, and the crude product was subjected to fast silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =10:1), the product 145mg was obtained, and the calculated yield was 72%.
[0033] Its main physical and chemical properties are as follows:
[0034] White solid, melting point: 166-168°C; 1 H NMR (400MHz, CDCl 3 ):δ8.02(br,1H),7.57(d,J=8.2Hz,2H),7.46(d,J=8.3Hz,2H),7.29(d,J=8.0Hz,2H),7.17(d ,J=8.1Hz,2H),3.77(s,3H),3.58(s,3H),2.43(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com