Antibacterial synergist, preparation method and uses thereof
A technology of uses, antibiotics, applied in the field of biomedicine, can solve problems such as macro-nephrotoxicity and neurotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0176] The preparation method of the compound of the present invention
[0177] The compound represented by the general formula (I) of the present invention can be prepared by the following method, but the conditions of the method, such as the amount of reactant, solvent, base, compound used, reaction temperature, reaction time required, etc. are not limited to the following Explanation. The compounds of the present invention can also optionally be conveniently prepared by combining various synthetic methods described in this specification or known in the art, such combinations can be easily performed by those skilled in the art to which the present invention pertains.
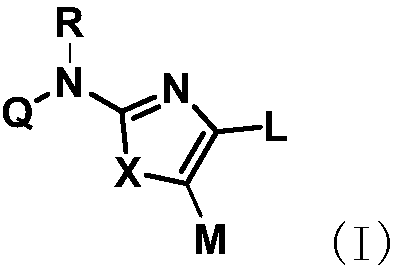
[0178] Compound shown in general formula (I) of the present invention can be synthesized by following method:
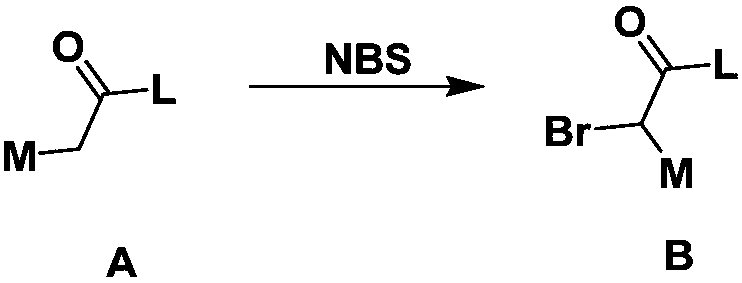
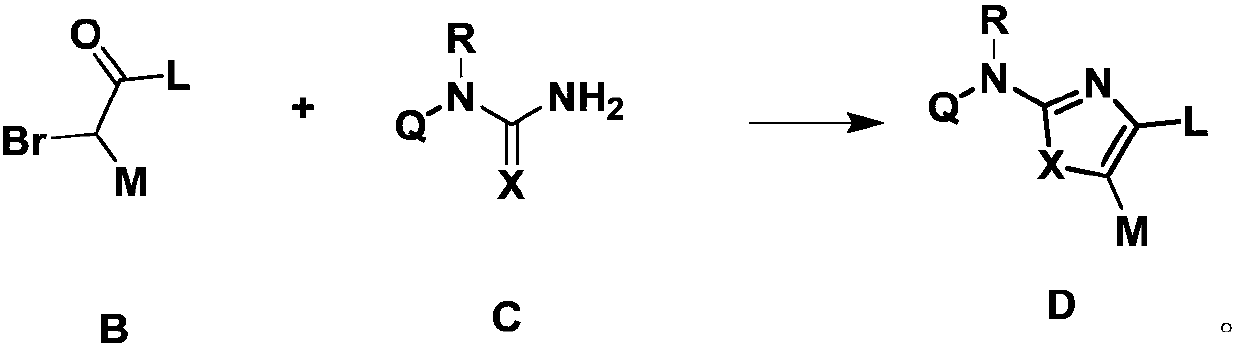
[0179] (1) In an inert solvent, the compound of formula A is reacted with NBS at a certain temperature (such as 40-80°C). After the reaction is completed, the compound of formula B is obtained by ...
Embodiment 1-57
[0204] Example 1-57: Preparation of Compounds
Embodiment 1
[0205] Example 1 2-(4-trifluoromethylphenylimino)-4-(4-methylphenyl)thiazole
[0206] 1.1 Preparation of α-bromo-4-methylacetophenone
[0207]
[0208] Add 10mmol 4-methylacetophenone and 11mmol N-bromosuccinimide (NBS) to a 100mL round bottom flask, dissolve in 35mL ethyl acetate, then add 1g Amberlyst 15 ion exchange resin as a catalyst, and heat up the reaction solution Reaction at 40°C. After the reaction was followed by TLC, the reaction solution was filtered to remove Amberlyst 15 ion exchange resin, the filtrate was spin-dried, and separated by column chromatography (eluent: petroleum ether / ethyl acetate) to obtain pale yellow crystals with a yield of 53%. 1 H NMR (400MHz, CDCl 3 )δ7.88(d, J=8.2Hz, 2H), 7.28(d, J=8.1Hz, 2H), 4.43(s, 2H), 2.42(s, 3H).
[0209] 1.2 Preparation of 2-(4-trifluoromethylphenylimino)-4-(4-methylphenyl)thiazole
[0210]
[0211] Add 1mmol N-(4-trifluoromethylphenyl)thiourea and 1.05mmol α-bromo-4-methylacetophenone to a 25mL eggplant-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com