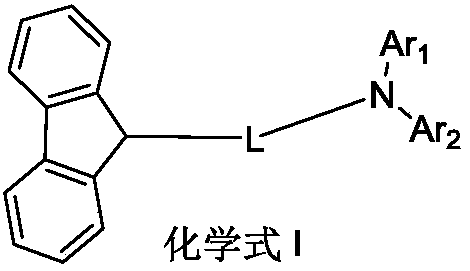
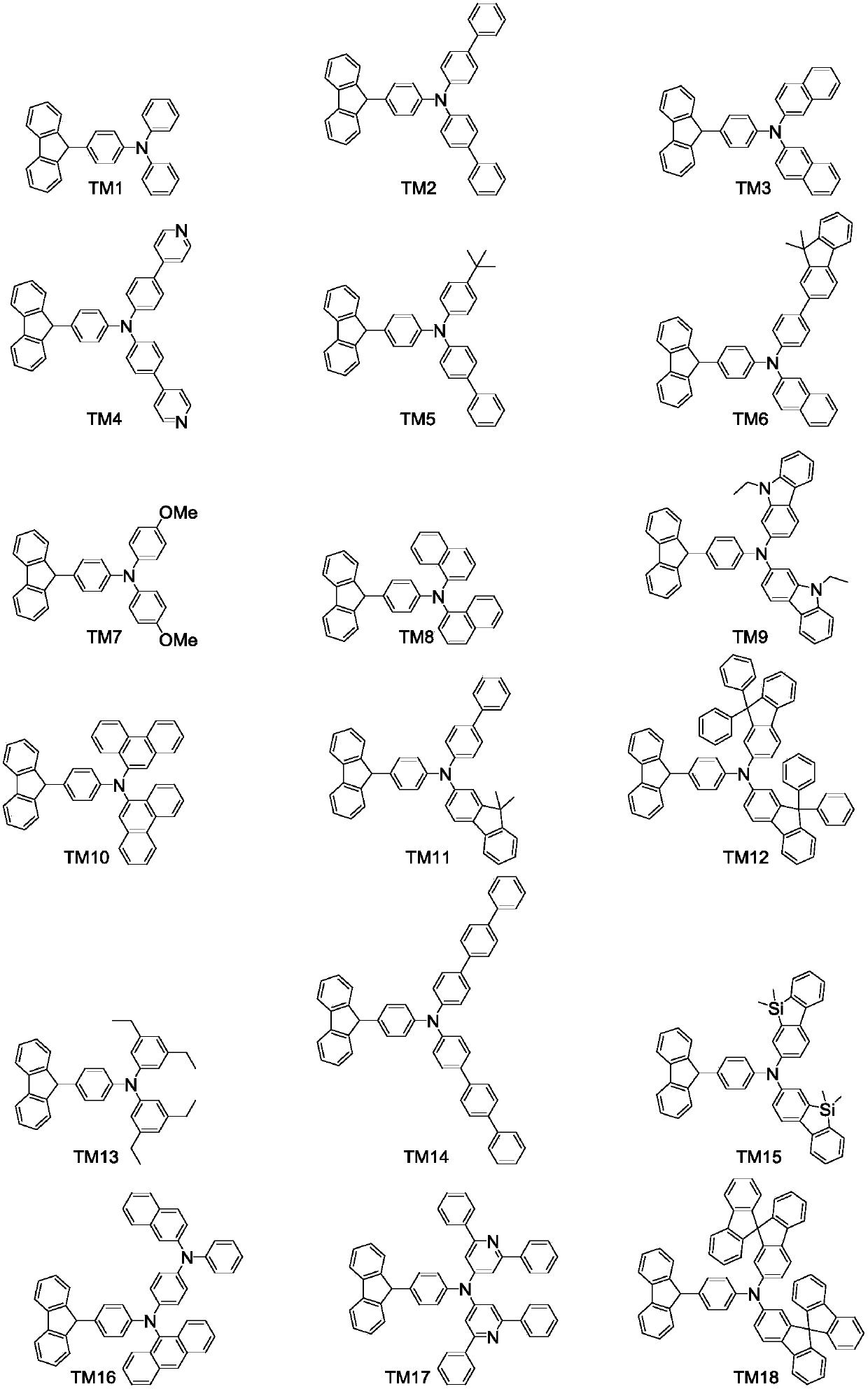
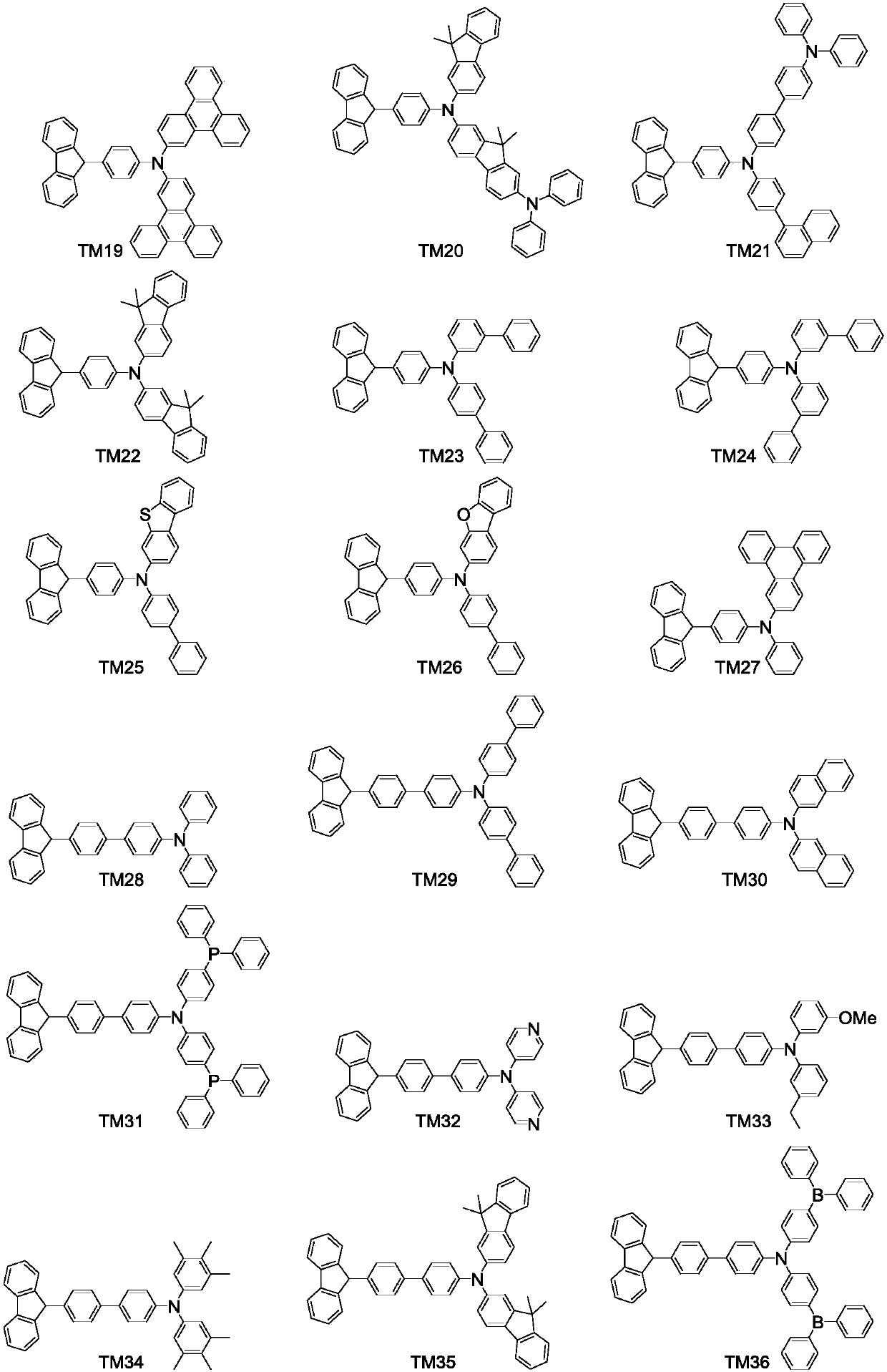
Fluorene derivative and organic luminescent device thereof
An organic light-emitting device and a technology of fluorene derivatives, which are applied in the field of fluorene derivatives and organic light-emitting devices, can solve problems such as inability to provide satisfactory light-emitting properties, and achieve good hole transport performance, high hole mobility, The effect of high luminous efficiency and luminous brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of Compound III-1
[0060]
[0061] Add compound I-1 (12.5g, 73.8mmol), compound II-1 (20.9g, 73.8mmol), t-BuONa (10.7g, 111mmol), Pd(OAc) in turn to the round bottom flask 2 (0.33g, 1.47mmol) and ultrasonically deoxygenated toluene (1.5L), refluxed overnight under nitrogen protection, treated the reaction solution with ethyl acetate and water after cooling, and the obtained organic layer was washed with MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain the crude product of compound III-1. With silica gel as the stationary phase and dichloromethane / hexane as the eluent, the crude product was subjected to column chromatography to obtain compound III-1 (18.7 g, 78 %). Mass spectrum m / z: theoretical value: 324.22; found value: 326.31. Theoretical element content (%)C 18 h 14 BrN: C, 66.68; H, 4.35; Br, 24.64; N, 4.32; Measured element content (%): C, 66.64; H, 4.38; Br, 24.62; N, 4.31. The above results confirmed that the ...
Embodiment 2
[0069] Preparation of Compound III-2
[0070]
[0071] Add compound I-2 (23.7g, 73.8mmol), compound II-2 (20.9g, 73.8mmol), t-BuONa (10.7g, 111mmol), Pd(OAc) in turn to the round bottom flask 2 (0.33g, 1.47mmol) and ultrasonically deoxygenated toluene (1.5L), refluxed overnight under nitrogen protection, treated the reaction solution with ethyl acetate and water after cooling, and the obtained organic layer was washed with MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain the crude product of compound III-2. With silica gel as the stationary phase and dichloromethane / hexane as the eluent, the crude product was subjected to column chromatography to obtain compound III-2 (28.8g, 82 %). Mass spectrum m / z: theoretical value: 476.41; found value: 478.54. Theoretical element content (%)C 30 h 22 BrN: C, 75.63; H, 4.65; Br, 16.77; N, 2.94; measured element content (%): C, 75.61; H, 4.68; Br, 16.75; N, 2.93. The above results confirmed that the o...
Embodiment 3
[0079] Preparation of Compound III-3
[0080]
[0081] Add compound I-3 (19.9g, 73.8mmol), compound II-3 (20.9g, 73.8mmol), t-BuONa (10.7g, 111mmol), Pd(OAc) in turn to the round bottom flask 2 (0.33g, 1.47mmol) and ultrasonically deoxygenated toluene (1.5L), refluxed overnight under nitrogen protection, treated the reaction solution with ethyl acetate and water after cooling, and the obtained organic layer was washed with MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain the crude product of compound III-3. With silica gel as the stationary phase and dichloromethane / hexane as the eluent, the crude product was subjected to column chromatography to obtain compound III-3 (23.5 g, 75 %). Mass spectrum m / z: theoretical value: 424.33; found value: 426.47. Theoretical element content (%)C 26 h 18 BrN: C, 73.59; H, 4.28; Br, 18.83; N, 3.30; measured element content (%): C, 73.54; H, 4.31; Br, 18.82; N, 3.27. The above results confirmed that the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com