Synthesis method of sodium sugammadex
A technology of sugammadex sodium and a synthetic method, which is applied in the field of preparation of muscle relaxant antagonist drugs, can solve the problems of inability to realize industrialization, large pollution, and low yield, and achieve easy industrial production, high yield, and good fat solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
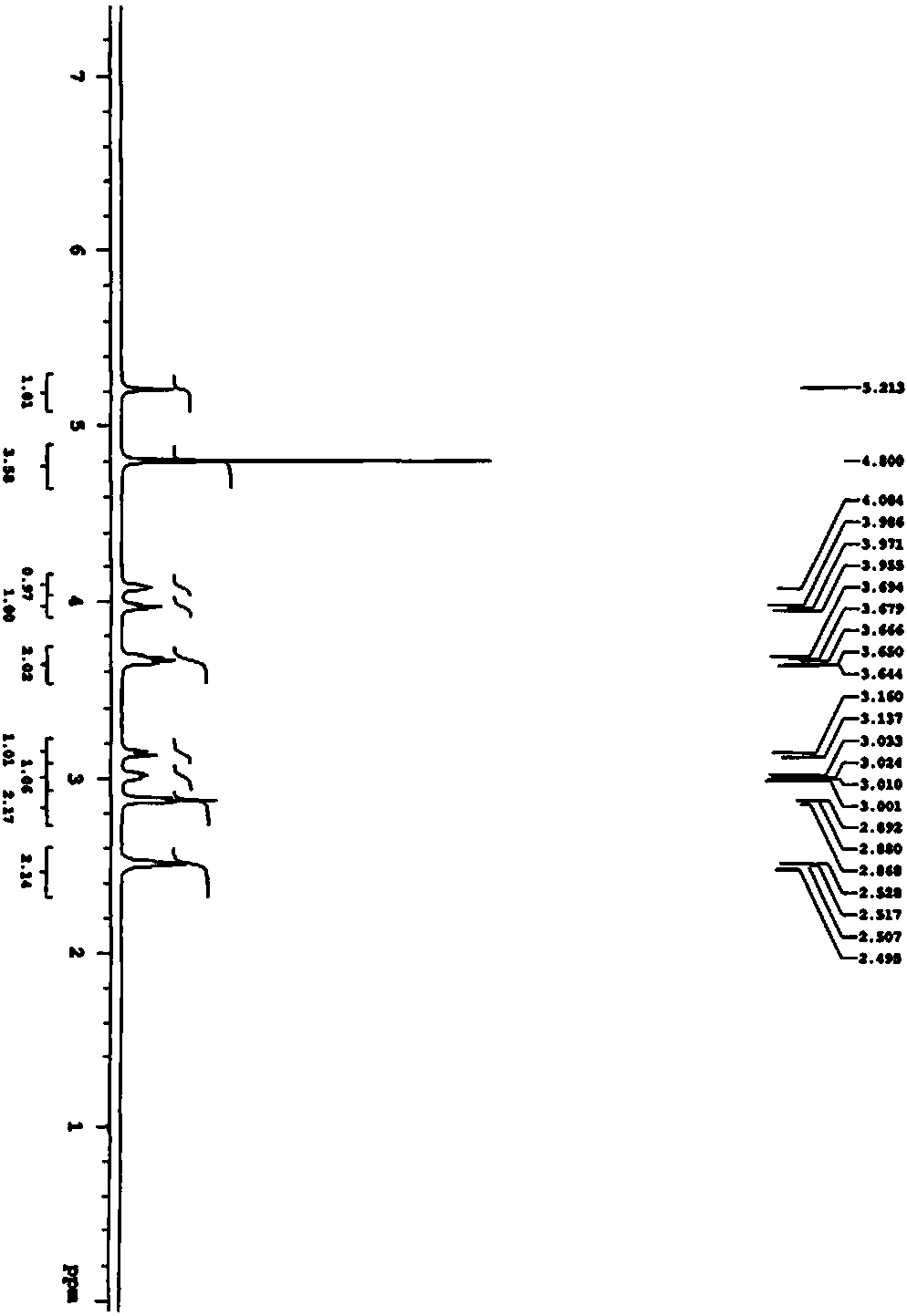
Examples
Embodiment 1
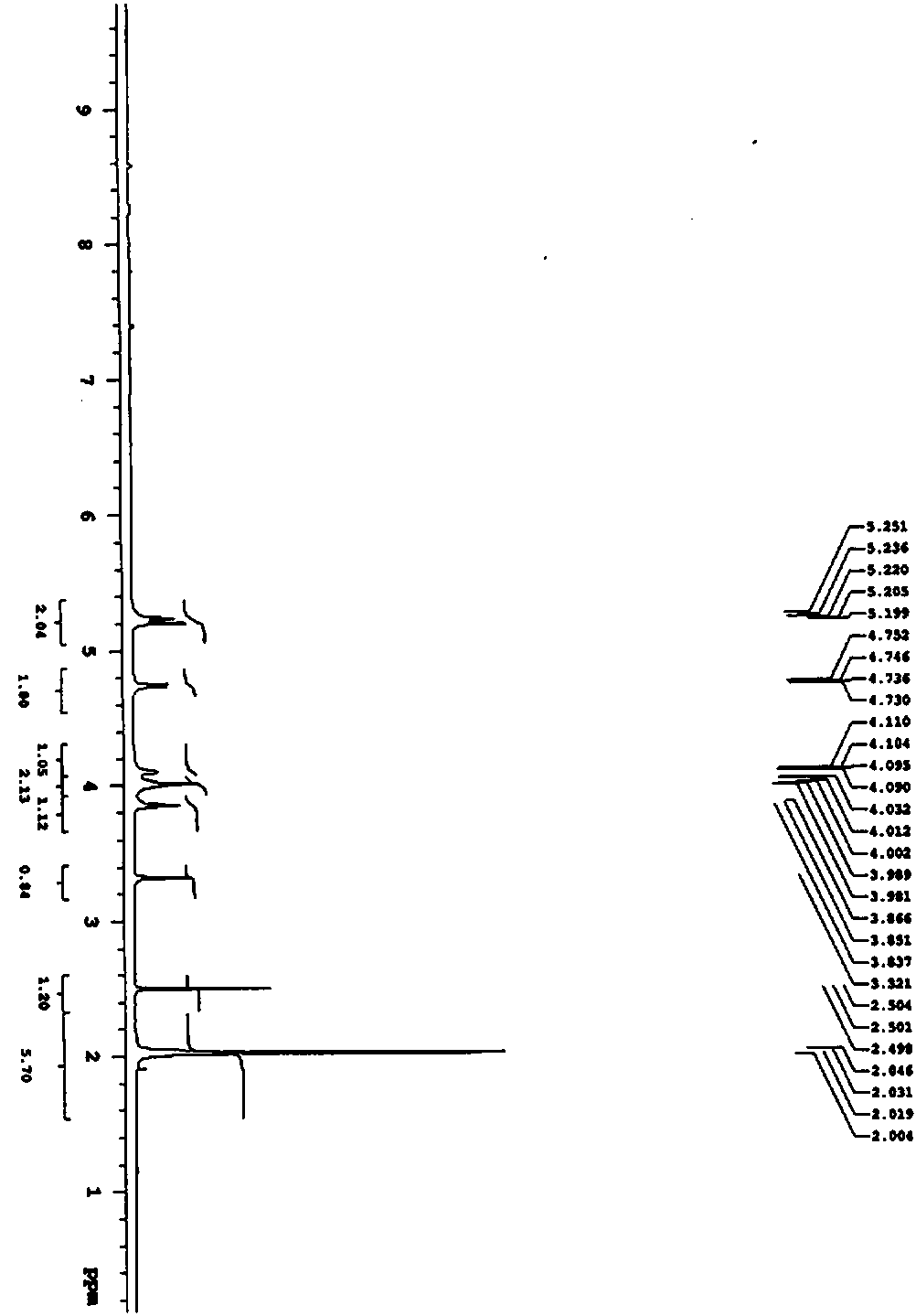
[0045] Synthesis of Compound III:
[0046] Add 100.00g of γ-cyclodextrin (dried at 105°C for 24h) and 1600mL of N,N-dimethylformamide into the reaction flask, stir to dissolve and raise the temperature to 65-75°C, drop 150.12g of methylsulfonate into the feed solution Acyl chloride, dropwise, keep stirring and react for 16-18h, then lower the temperature to 0°C, add sodium methoxide-methanol solution to the system to adjust the pH>10, pour the reaction solution into 3200mL water and stir for 30min, centrifuge, wash the filter cake with water, and dry to obtain 103.5 g of white solid compound III. Yield 99%, LC / MS ES - -Mass-to-core ratio of API [M-H] - = 1443.40.
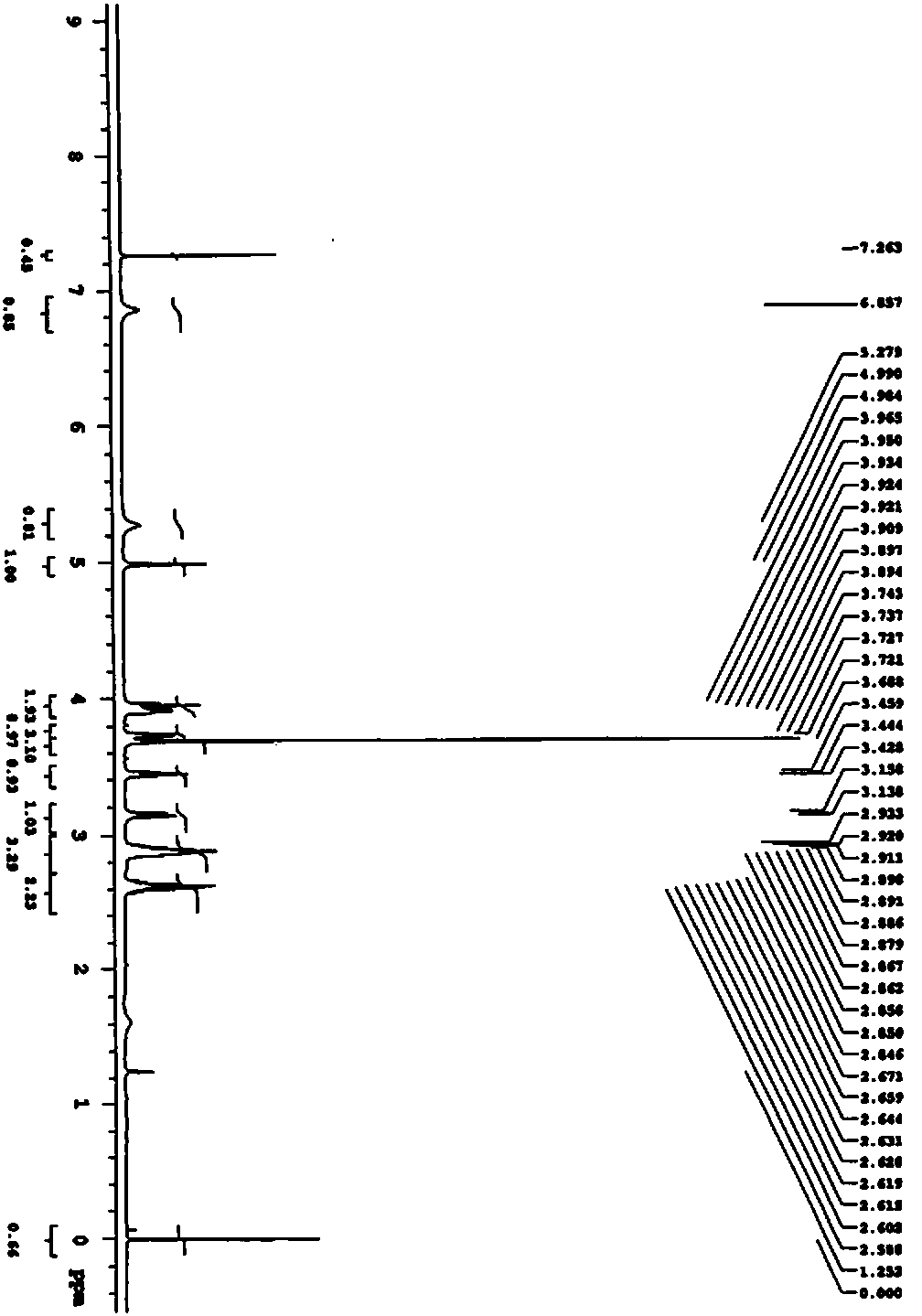
[0047] Synthesis of Compound IV:
[0048]Add 300mL of pyridine and 200mL of acetic anhydride to 100.00g of compound III, stir and heat up to 60°C, keep warm at 55-65°C and stir for 6-8h, monitor by TLC, and cool down to room temperature after the raw material point disappears. Add 1200mL water to the system, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com