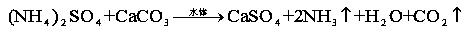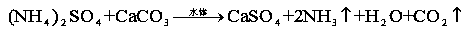Novel application of solid ammonium sulfate and calcium carbonate reaction in water ammonia nitrogen treatment
A technology of calcium carbonate and ammonium sulfate, applied in water/sewage treatment, water/sludge/sewage treatment, water pollutants, etc., can solve the problems of poor effect and high cost, and achieve the reduction of ammonia nitrogen content, low cost and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Accurately weigh 50g of calcium carbonate and 15g of ammonium sulfate and mix them in a three-necked flask with a specification of 250ml. Connect a separatory funnel with a specification of 50ml and 2 / 3 water at the 1st port, and connect a constant temperature electric stirrer at the 2nd port, and drip water while Stir, let it fully react at room temperature, connect the catheter at the 3 port to export the reaction gas, and introduce the gas into the clarified lime water, the lime water becomes turbid, which proves that carbon dioxide gas is produced, put the wet red test paper on the catheter port, the test paper becomes Blue, proving that ammonia gas is produced. The reaction equation is as follows:
[0017]
Embodiment 2
[0019] Accurately weigh 50g of calcium carbonate and 15g of ammonium sulfate and mix them in a 200ml beaker, stir with a constant temperature stirrer, spray water while stirring, ensure that the stirring speed is 350r / min and the temperature is 45°C, and record the reaction within 5h. amount of gas. The reaction analysis data of different time periods are shown in Table 1.
[0020] Table 1 Analysis data of different time periods
[0021] time h
[0022] The solid-liquid reaction of ammonium sulfate calcium carbonate will stop the reaction quickly due to the package of calcium sulfate. The above reaction data show that if there is friction between solids, the reaction can always be carried out. With the increase of reaction time, the degree of reaction increases. If the conditions required for the reaction are guaranteed, the reaction degree and the reaction time are basically in a linear relationship.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com