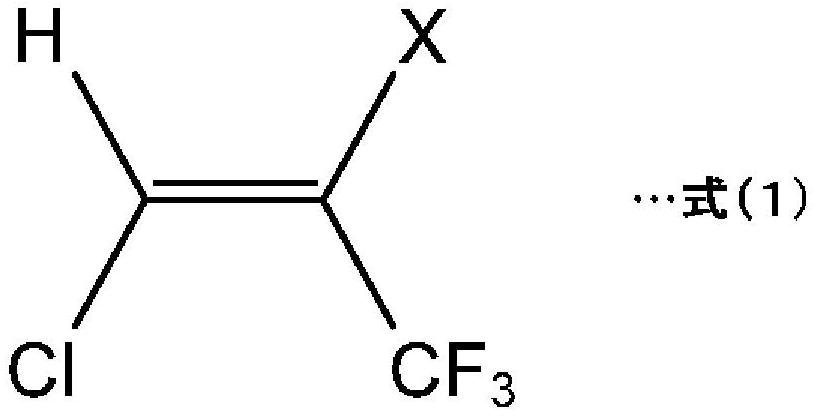
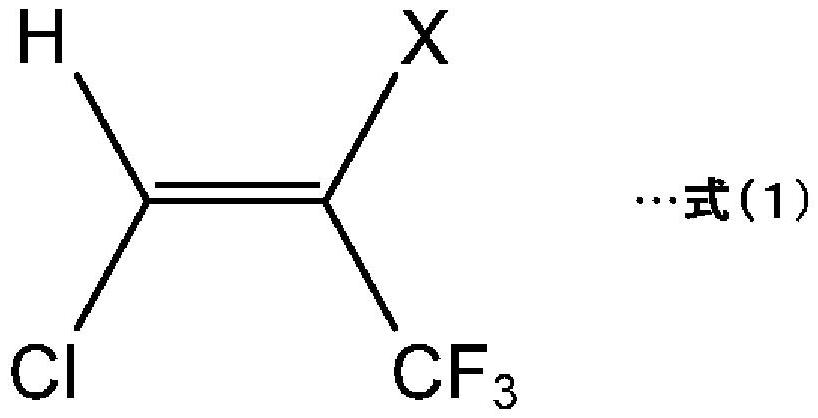
Method for producing hydrochlorofluoroolefin and method for producing 2,3,3,3-tetrafluoropropene
A technology for hydrochlorofluoroolefins and a manufacturing method, which is applied to the preparation of halogenated hydrocarbons, organic chemistry methods, chemical instruments and methods, etc., can solve the problems that only one geometric isomer is not suitable for use, and achieves the suppression of by-products. Generating, Efficiently Manufactured Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0115] Examples are described below, but the present invention is not limited thereto. Examples 1-20 and Examples 24-25 are examples, and Examples 21-23 are reference examples.
[0116] (catalyst preparation example 1)
[0117] 81.5 mL of chromium-magnesium composite oxide catalyst (Cr 2 o 3 : 98% by mass, MgO: 2% by mass, AG-23, Sakai Chemical Industry Co., Ltd. (Sakai Chemical)), nitrogen (N 2 ) while heating to 200°C. The temperature was maintained to dry the catalyst until no outflow of water was observed at the outlet of the reactor. After drying of the catalyst, the N 2 The gas and HCFC-22 circulate together in the reactor, and when the hot spot caused by the activation of the filled catalyst reaches the outlet end of the reactor, the temperature of the reactor is raised to 250°C, and the state is maintained for 8 hours, and the catalyst activation treatment is carried out , Catalyst 1 was obtained.
[0118] (catalyst preparation example 2)
[0119] Catalyst 2 wa...
Synthetic example 1
[0132] (Synthesis Example 1: Production of HCFO-1224yd)
[0133] HCFO-1224yd was produced by the same method as the method described in Patent Document 3. In a tubular reactor made of stainless steel (SUS316), palladium-supported activated carbon obtained by supporting palladium on activated carbon was filled to form a catalyst layer. Thereafter, the reactor was kept at 80° C. in a heating furnace, and hydrogen (H 2 ) / CFO-1214ya molar ratio = 1 / 1 mixed gas mixture of CFO-1214ya and hydrogen. At this time, the above mixed gas was supplied into the reactor so that the residence time of the mixed gas of CFO-1214ya and hydrogen in the catalyst layer was 25 seconds.
[0134] The outlet gas obtained from the above reactor was passed through a 10% by mass potassium hydroxide (KOH) aqueous solution to remove acidic components, and then passed through a dehydration tower filled with synthetic zeolite (Molecular Sieve 4A (Molecular Sieve 4A)). dehydration. The dehydrated outlet gas ...
example 1
[0136] A tubular reactor made of stainless steel (SUS316) with an inner diameter of 23.4 mm and a height of 400 mm (hereinafter also referred to as a reactor) was filled with 81.5 mL of the catalyst 1 . With the raw material composition A of 27.9NmL / min and the nitrogen (N 2 ) ratio, and preheated to 50°C through a heating furnace. Thereafter, a mixed gas of the preheated raw material composition A and nitrogen was circulated in the reactor kept at 50° C. by a heating furnace under conditions close to atmospheric pressure. The outlet gas was collected from the reactor and analyzed by GC (gas chromatography). As a result, the composition of the outlet gas was 88.2 (GC area %) for HCFO-1224yd(Z) in terms of area percentage in GC (GC area %) ), HCFO-1224yd(E) was 11.1% (GC area %). In addition, the reaction time (the residence time of the raw material composition A in the reactor) was 49.5 seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com