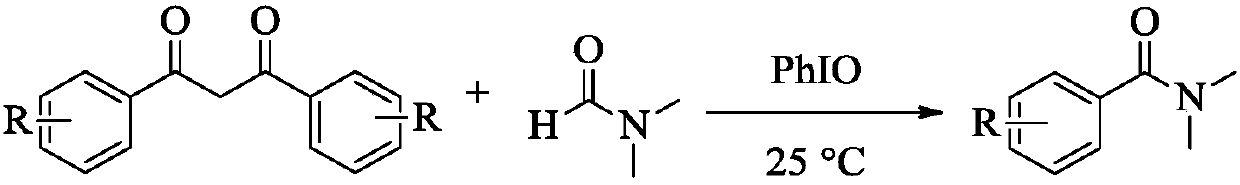
Preparation method of N,N-dimethyl benzamide
A technology of dimethylbenzamide and dimethylformamide, which is applied in the field of metal-free catalytic synthesis of N,N-dimethylbenzamide, and can solve problems such as limitations, reaction conditions to be improved, and high reaction temperature , to achieve the effect of high selectivity, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0009] In the present invention, 0.2mmol dibenzoylmethane, 0.4mmol iodosobenzene, 2mL N,N-dimethylformamide solution and No. 5 magnet are added in sequence in the reaction tube, and the reaction tube is placed in 25°C oil Heat the reaction in a bath for 24 hours. After the reaction, pour the reaction solution into a separatory funnel, add 10 mL of ethyl acetate, and carry out back extraction three times with 15 mL of distilled water. After collecting the organic phase, the crude product is obtained by distillation under reduced pressure. The obtained crude product The light yellow pure product was obtained after separation by column chromatography.
[0010] General reaction formula of the present invention is:
[0011]
[0012] The reactor is a micro reaction tube, and the micro reaction tube is placed in an oil bath at 25° C. for stirring.
[0013] The bottom end of the micro reaction tube is immersed in the silicone oil, and the immersion depth is that the height of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com