Preparation method of PCV2, PRRSV and mycoplasma hyopneumoniae triple inactivated vaccine
A technology of Mycoplasma hyopneumoniae and inactivated vaccine, applied in the field of veterinary biological products, can solve problems such as shortage, and achieve the effects of ensuring uniformity, improving immune effect and production performance, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of PCV2, PRRSV, mycoplasma hyopneumoniae triple inactivated vaccine, comprising the following steps:
[0035] 1. Production seed preparation
[0036] 1.1 Porcine circovirus production seed preparation
[0037] 1.1.1 Cell Propagation
[0038] Resuscitate PK15 cells, add cell nutrient solution, culture at 37°C for 24-48 hours, and pass passage after forming a good cell monolayer. When passaging, discard the nutrient solution, add EDTA-trypsin digestion solution, act for several minutes, digest and disperse evenly to make cell suspension. Then it was divided into cell culture flasks, incubated at 37°C for 24 hours, and tested for sterility, mycoplasma and exogenous virus according to the appendix of the current "Chinese Veterinary Pharmacopoeia", which should meet the requirements.
[0039] 1.1.2 Breeding of poisonous seeds
[0040] Inoculate PK-15 cells into cell culture medium, adjust the concentration to 200,000-300,000 / mL, and incubate with PCV...
Embodiment 2
[0080] The safety research of the vaccine prepared in embodiment 2 embodiment 1
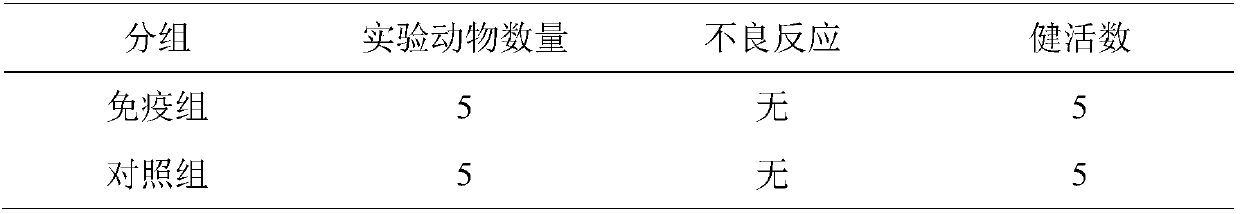
[0081] Get the vaccine in Example 1, mark the head portion (2mL / head portion) by the bottle label, intramuscularly inject 5 portions after weaning 1.5-2 months old pigs (negative swine fever neutralizing antibody, pig PCV2ELISA antibody negative, pig PRRSVELISA Antibody negative, Mycoplasma swine ELISA antibody negative) 5 heads, 5 heads in the control group were not immunized, observed daily for 21 days, there should be no local or systemic adverse reactions caused by the injection of the vaccine. The results are shown in Table 1.
[0082] Table 1 Safety study results
[0083]
[0084] The safety test results showed that the triple inactivated vaccine of porcine circovirus type 2, porcine reproductive and respiratory syndrome virus and mycoplasma hyopneumoniae was safe for weaned piglets.
Embodiment 3
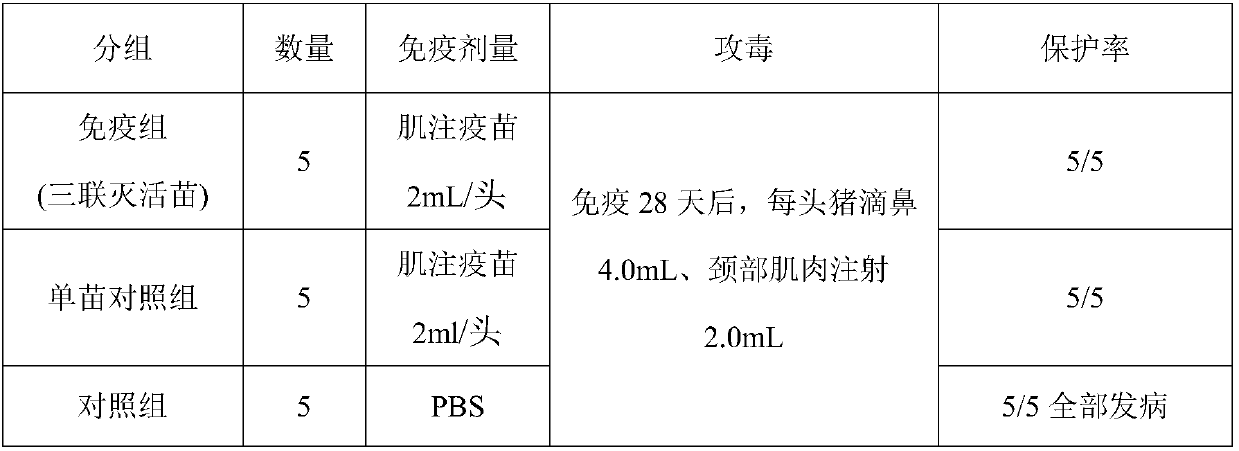
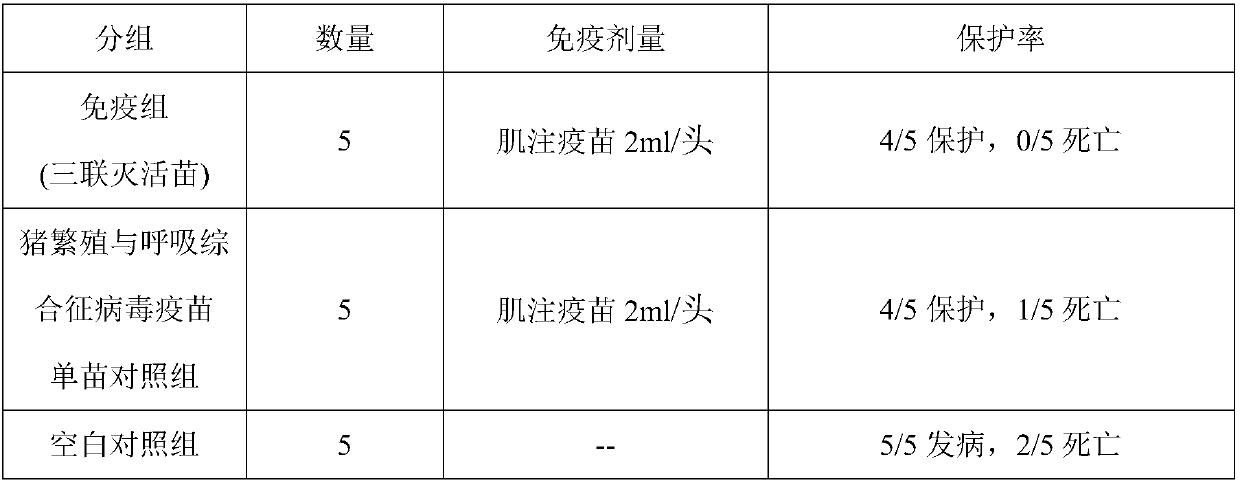
[0085] Example 3 Vaccine Vaccine Efficacy Research Prepared in Example 1
[0086] 1.1 Porcine circovirus type 2 vaccine immunization and challenge
[0087] Fifteen healthy susceptible piglets with negative PCV2 ELISA antibody, PCV2 antigen negative and PRRSV ELISA antibody negative (commercially registered ELISA antibody kit detection) aged 14-21 days were randomly divided into 3 groups, 5 pigs in each group. The first group is the triple inactivated vaccine immunization group of porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and mycoplasma hyopneumoniae, intramuscularly injecting vaccine 2mL / head; the second group is the PCV2 vaccine immunization group, and the third group is non-inactivated Blank control was inoculated. The virus was challenged 28 days after vaccination, and each pig was weighed before the challenge. Group 1 was inoculated with PCV2 (10 7.0 TCID 50 4.0 mL / ml), wherein each pig was intranasally dripped with 4.0 mL, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com