Electrochemical fluorination preparation method of hydrogen fluoride ether
A hydrofluoroether and electrochemical technology, applied in the field of electrochemical fluorination preparation of hydrofluoroether, can solve problems such as low yield, and achieve the effects of high yield, high purity and less product impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
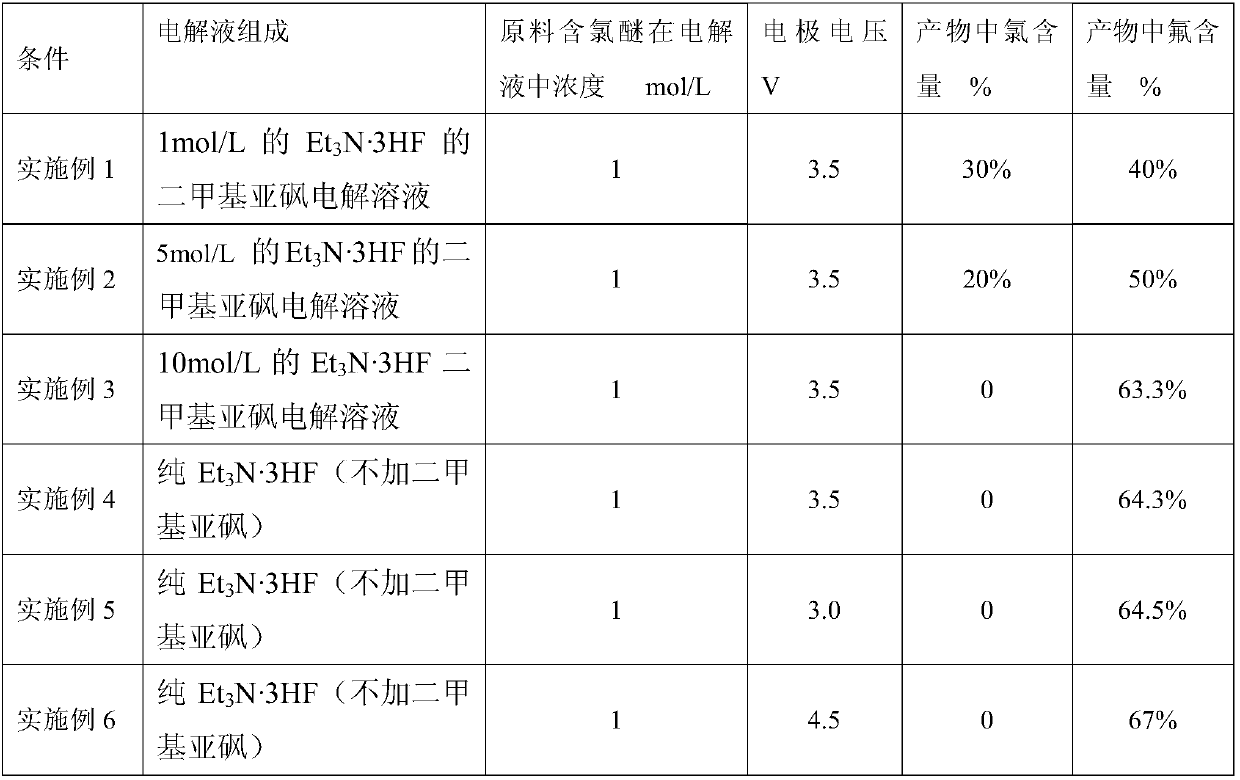
[0027] 1mol / L Et 3 Dimethyl sulfoxide electrolytic solution of N 3HF as electrolyte, CFCl 2 -CF 2 -O-CH 3The concentration in the electrolyte is 1mol / L, and 1L of the electrolyte containing the product is added to a 2.5L electrochemical fluorination electrolyzer. The electrochemical fluorination electrolytic cell is equipped with a pair of platinum electrode sheets of about 2 square decimeters, placed in parallel to maintain constant potential electrolysis, the current is adjusted to 50mA, the electrode potential is set to 3.5V, the reaction temperature is 25 ° C, nitrogen Carried out under the normal pressure of the protection formation. After the electrolysis is completed, add 500ml of water to the electrolyte, then extract it with ether for 5 times, take the ether phase and dry it with anhydrous sodium sulfate, filter under reduced pressure to obtain the filtrate, which is the product, and take a small amount to test for chlorine and fluorine elements , and the results ...
Embodiment 2-7
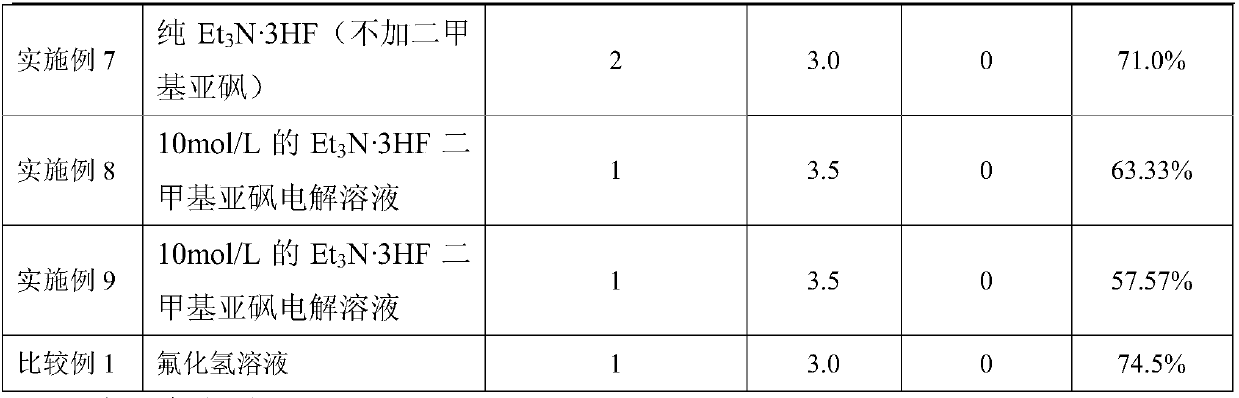
[0029] Adopt the method identical with embodiment 1 to carry out experiment, but change the Et in the electrolytic solution 3 The concentration and electrode voltage of N·3HF, conditions and results are listed in Table 1.
Embodiment 8-9
[0031] Adopt the method identical with embodiment 1 to carry out experiment, but change raw material type, condition and result are listed in table 1, embodiment 8 adopts raw material CFCl 2 -CFCl-O-CH 3 , embodiment 9 adopts raw material CHCl 2 -CF 2 -O-CH 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com