Composite lithium iron phosphate anode material and preparation method thereof
A composite cathode material, lithium iron phosphate technology, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve the problems of low conductivity and unsatisfactory rate performance of lithium iron phosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
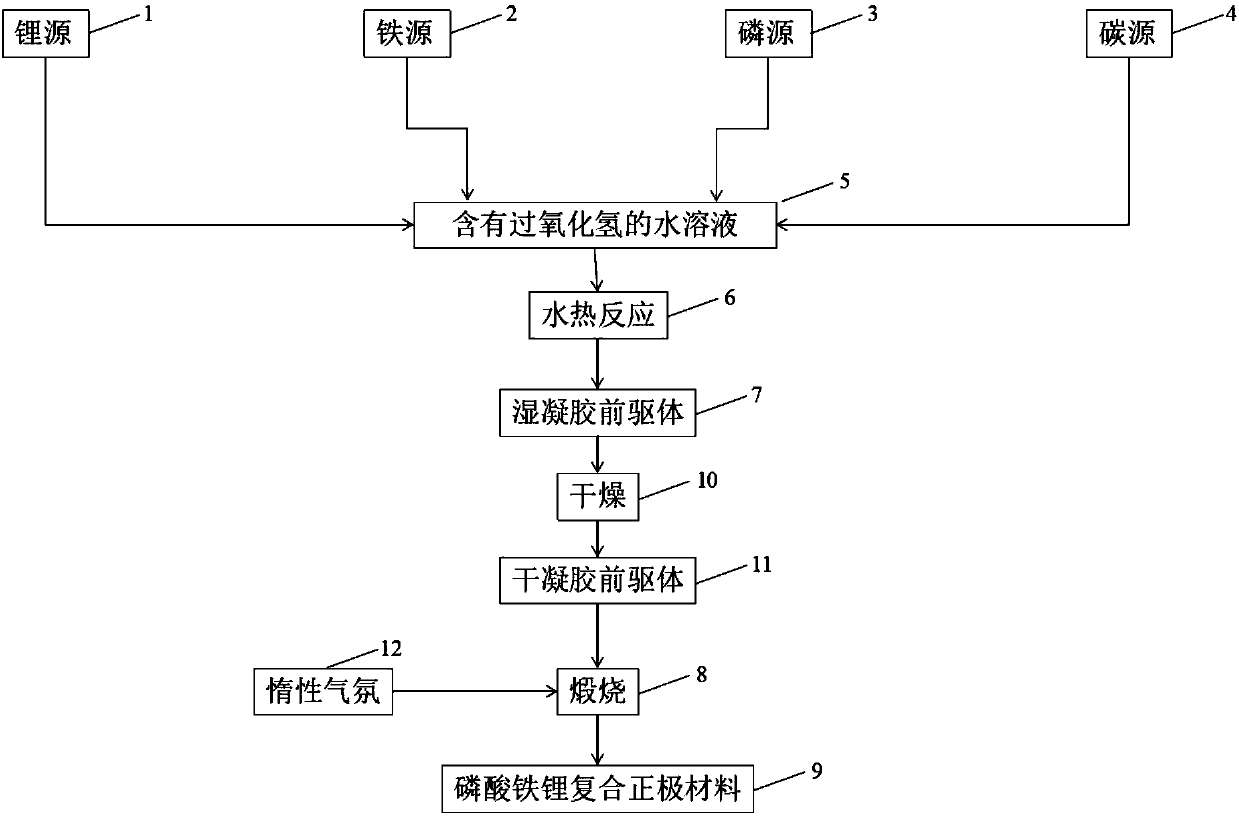
[0031] The invention provides a lithium iron phosphate composite positive electrode material, the preparation method of embodiment 1 is as follows: weigh 37mg (0.5mmol) lithium carbonate (Li 2 CO 3 ), 144mg (1mmol) ferrous oxalate (FeC 2 o 4 ), 115mg (1mmol) ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) and 31.6mg (20wt.%) citric acid (C 6 h 8 o 7 ) was added to 50mL deionized water and 5mL H 2 o 2 After it was completely dissolved, it was put into a stainless steel reaction kettle lined with polytetrafluoroethylene, and hydrothermally reacted at 180°C for 6h. After the reaction is completed, the obtained wet gel precursor is taken out, freeze-dried for 12 hours under vacuum, and finally the dry gel precursor is put into an argon atmosphere and calcined at 750° C. for 8 hours to obtain the lithium iron phosphate composite cathode material of the present invention .
[0032] Lithium source 1 is lithium carbonate, iron source 2 is ferrous oxalate, phosphorus source 3...
Embodiment 2
[0038] see figure 1 , the invention provides a kind of lithium iron phosphate composite cathode material, the preparation method of embodiment 2 is as follows: Weigh 37mg (0.5mmol) lithium carbonate (Li 2 CO 3 ), 144mg (1mmol) ferrous oxalate (FeC 2 o 4 ), 115mg (1mmol) ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) and 63.2mg (40wt.%) citric acid (C 6 h 8 o 7 ) was added to 50mL deionized water and 5mL H 2 o 2 After being completely dissolved, put it into a stainless steel reaction kettle lined with polytetrafluoroethylene, and conduct a hydrothermal reaction at 180°C for 6 hours. After the reaction was completed, the obtained gel precursor was taken out, freeze-dried under vacuum for 12 hours, and finally the dry gel was put into a nitrogen atmosphere and calcined at 750° C. for 8 hours to obtain the lithium iron phosphate composite cathode material of the present invention.
Embodiment 3
[0040] see figure 1 , the present invention provides a lithium iron phosphate composite positive electrode material, the preparation method of embodiment 3 is as follows: respectively weigh 24mg (1mmol) lithium hydroxide (LiOH), 152mg (1mmol) iron phosphate (FeSO 4 ), 115mg (1mmol) ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) and 79mg (50wt.%) citric acid (C 6 h 8 o 7 ) was added to 50mL deionized water and 5mL H 2 o 2 After it was completely dissolved, it was put into a stainless steel reactor lined with polytetrafluoroethylene, and hydrothermally reacted at 160°C for 6h. After the reaction is completed, the obtained wet gel precursor is taken out, freeze-dried under vacuum for 12 hours to obtain a dry gel precursor, and finally the dry gel precursor is put into a hydrogen atmosphere and calcined at 750°C for 8 hours to obtain the present invention. Lithium iron phosphate composite cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com