Multi-trace-element V pharmaceutical composition and uses thereof
A technology of trace elements and compositions, applied in the field of medicine, can solve problems such as adverse consequences, poor patient tolerance or clinical accidents, and uncomfortable patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] The preparation of embodiment 1 multiple trace element pharmaceutical composition injection
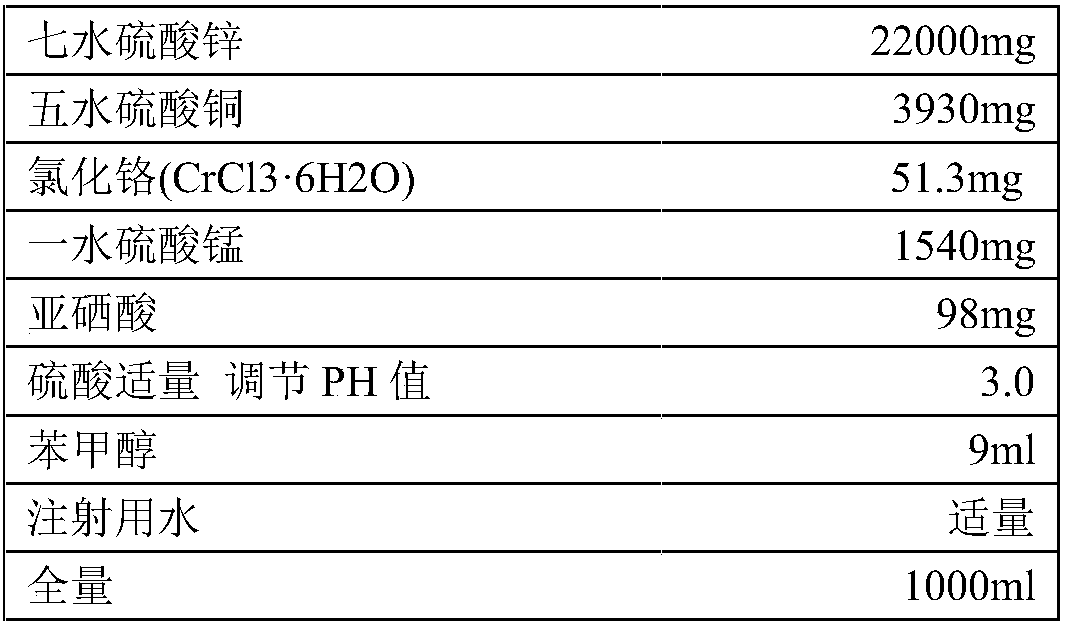
[0095] Prescription: zinc gluconate hydrate 34862mg (calculated as zinc gluconate anhydrate)
[0096]
[0097] Appropriate amount of 2M gluconic acid solution and 2M sodium citrate solution
[0098] Appropriate amount of water for injection
[0099] Add water for injection to the full volume of 1000ml
[0100] Preparation process: Weigh or prepare raw and auxiliary materials according to the prescription quantity, step 1, stir and dissolve the prescription quantity of sodium gluconate, sorbitol, taurine, and sodium pantothenate with an appropriate amount of fresh water for injection, and acidify the pH with gluconic acid solution value to 3.8; step 2, chromium chloride 6 hydrate, copper sulfate pentahydrate, and manganese sulfate 1 hydrate are respectively acidified with gluconic acid solution and fresh water for injection is stirred and dissolved, and the solution is kept...
Embodiment 2
[0102] The preparation of embodiment 2 multiple trace element pharmaceutical composition injections
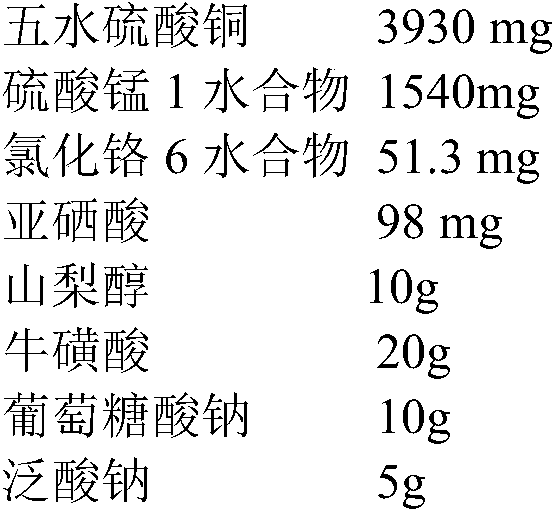
[0103] Prescription: zinc gluconate trihydrate 38997mg, chromium chloride hexahydrate 51.3mg, copper sulfate pentahydrate 3930mg, manganese sulfate monohydrate 1540mg, sodium selenite 5hydrate 145mg, taurine 20g, sorbitol 50g, Sodium gluconate 10g, L-sodium pantothenate 3g, appropriate amount of 2M gluconic acid solution and 2M sodium lactate solution, appropriate amount of water for injection, add water for injection to the full amount of 1000ml
[0104] Preparation process: Weigh or prepare raw and auxiliary materials according to the prescription quantity, step 1, stir and dissolve the prescription quantity of taurine, sodium gluconate, sorbitol, and L-sodium pantothenate with an appropriate amount of fresh water for injection, acidify the pH of the gluconic acid solution value to 3.8; step 2, chromium chloride 6 hydrate, copper sulfate pentahydrate, manganese sulfate 1 hyd...
Embodiment 3
[0105] The preparation of embodiment 3 multiple trace element pharmaceutical composition injections
[0106] Prescription: Zinc gluconate trihydrate 38997.3mg, chromium chloride hexahydrate 51.3mg, copper sulfate pentahydrate 3930mg, manganese sulfate monohydrate 1540mg, sodium selenite 5hydrate 145.1mg, taurine 30g, sorbitol 20g, glycine 20g, sodium gluconate 12g, appropriate amount of 2M gluconic acid solution and 2M sodium hydroxide solution, appropriate amount of 5M sodium hydroxide solution, appropriate amount of water for injection, add water for injection to the full amount of 1000ml
[0107] Preparation process: Weigh or prepare raw and auxiliary materials according to the prescription quantity. Step 1. Stir and dissolve the prescription quantity of taurine, sorbitol, glycine, and sodium gluconate with an appropriate amount of fresh water for injection. Jointly adjust the pH value of the solution to 3.9; Step 2, stir and dissolve the water for injection acidified with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com