Drug for preventing and treating alcoholic liver disease
A technology of alcoholic liver disease and medicament, which is applied in the fields of acute alcoholic liver injury and alcoholic liver fibrosis, prevention and treatment of alcoholic liver disease, and can solve the problems of curative effect blocking progress of alcoholic liver disease, so as to achieve prevention and treatment Alcoholic liver fibrosis symptoms, simple raw material components, and the effect of improving liver cell swelling and fatty degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
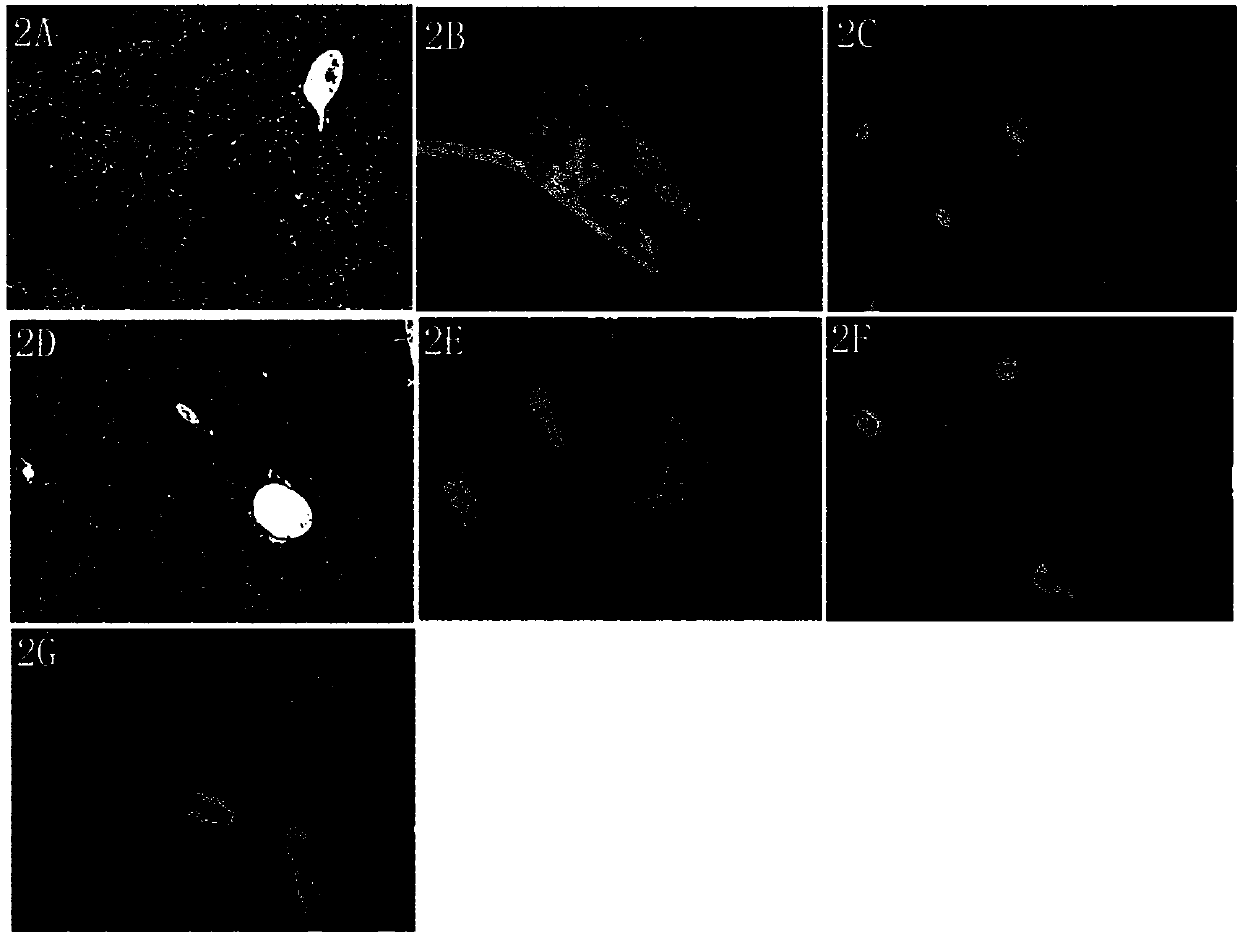
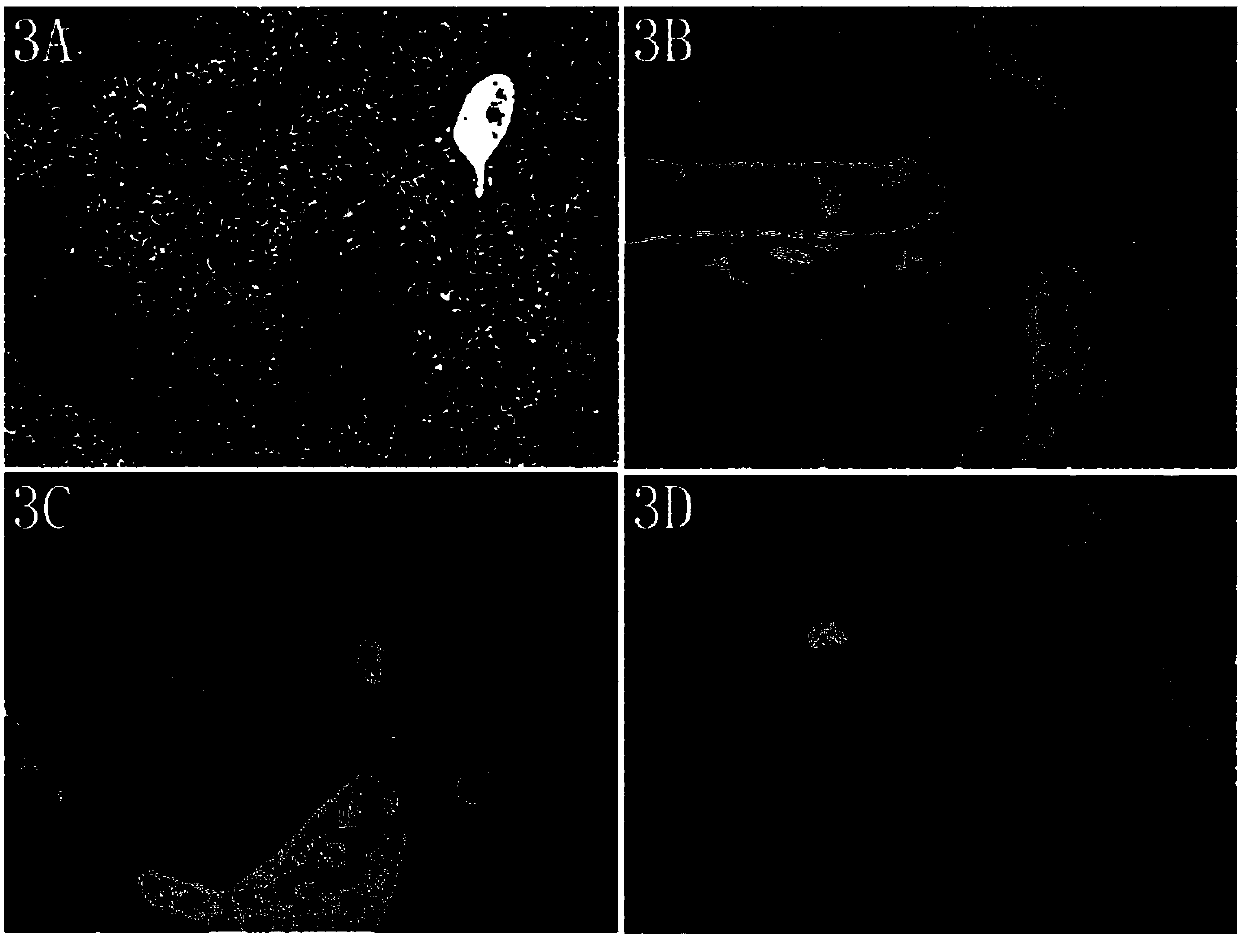
Image
Examples
preparation example Construction
[0056] Another object of the present invention is to provide a preparation method of a medicament for preventing and treating alcoholic liver disease, the preparation is a liquid medicament, and the preparation method comprises the following steps:
[0057] Step (1), preparing the Lactobacillus rhamnosus supernatant;
[0058] In step (2), vitamin E is added to the supernatant of Lactobacillus rhamnosus, preferably sodium tanshinone IIA sulfonate is also added.
[0059] In step (1), preparing the Lactobacillus rhamnosus supernatant comprises the following steps:
[0060] Step (1-1) activation: take 3% inoculum of Lactobacillus rhamnosus and inoculate it into MRS liquid medium (weigh 14.4g MRS broth powder and dissolve it in 300ml distilled water, store at 4°C after autoclaving), 37 Cultivate with shaking at ℃ for 20-30 hours, so that the strains in the liquid medium reach the set concentration;
[0061] Step (1-2) purification: take the bacterial solution and inoculate it in ...
Embodiment 1
[0074] The composition of the prescription is as follows: 1 mL of Lactobacillus rhamnosus supernatant and 100 mg of vitamin E.
[0075] Adopt following preparation method to obtain liquid medicament:
[0076] Step (1), preparing the Lactobacillus rhamnosus supernatant;
[0077]Activation: Take 3% inoculum of Lactobacillus rhamnosus and inoculate it into 5mL MRS liquid medium (weigh 14.4g MRS broth powder and dissolve it in 300ml distilled water, store at 4°C after autoclaving), shake and culture at 37°C for 24 hours , so that the strains in the liquid medium reach A600 of 1.380-1.400;
[0078] Purification: Inoculate 150 μL of bacterial liquid into MRS solid medium and incubate at 37°C for 24 hours;
[0079] Proliferation: Inoculate pale white circular flat single colonies with a diameter of 1.8-2mm into 4-6mL MRS liquid culture medium, culture at 37°C for 24 hours, and screen for highly active bacteria;
[0080] Extraction: Centrifuge the highly active bacterial solution a...
Embodiment 2
[0083] The composition of the prescription is as follows: 1 mL of supernatant of Lactobacillus rhamnosus, 100 mg of vitamin E and 50 mg of sodium tanshinone ⅡA sulfonate.
[0084] Adopt following preparation method to obtain liquid medicament:
[0085] Step (1), prepare Lactobacillus rhamnosus supernatant, method is the same as embodiment 1;
[0086] In step (2), 100 mg of vitamin E and 50 mg of sodium tanshinone IIA sulfonate are added to 1 mL of the supernatant of Lactobacillus rhamnosus to obtain a liquid medicine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com