Synthesis method of a-d-a type organic optoelectronic small molecules based on perylene diimide and pentacene
A technology of perylene diimide and A-D-A, which is applied in the field of synthesis of A-D-A type organic optoelectronic small molecules, can solve the problems of lack of application and principle, and achieve the effect of optimizing efficiency and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthetic method of the A-D-A type organic optoelectronic small molecule based on perylene diimide adopts the following steps:
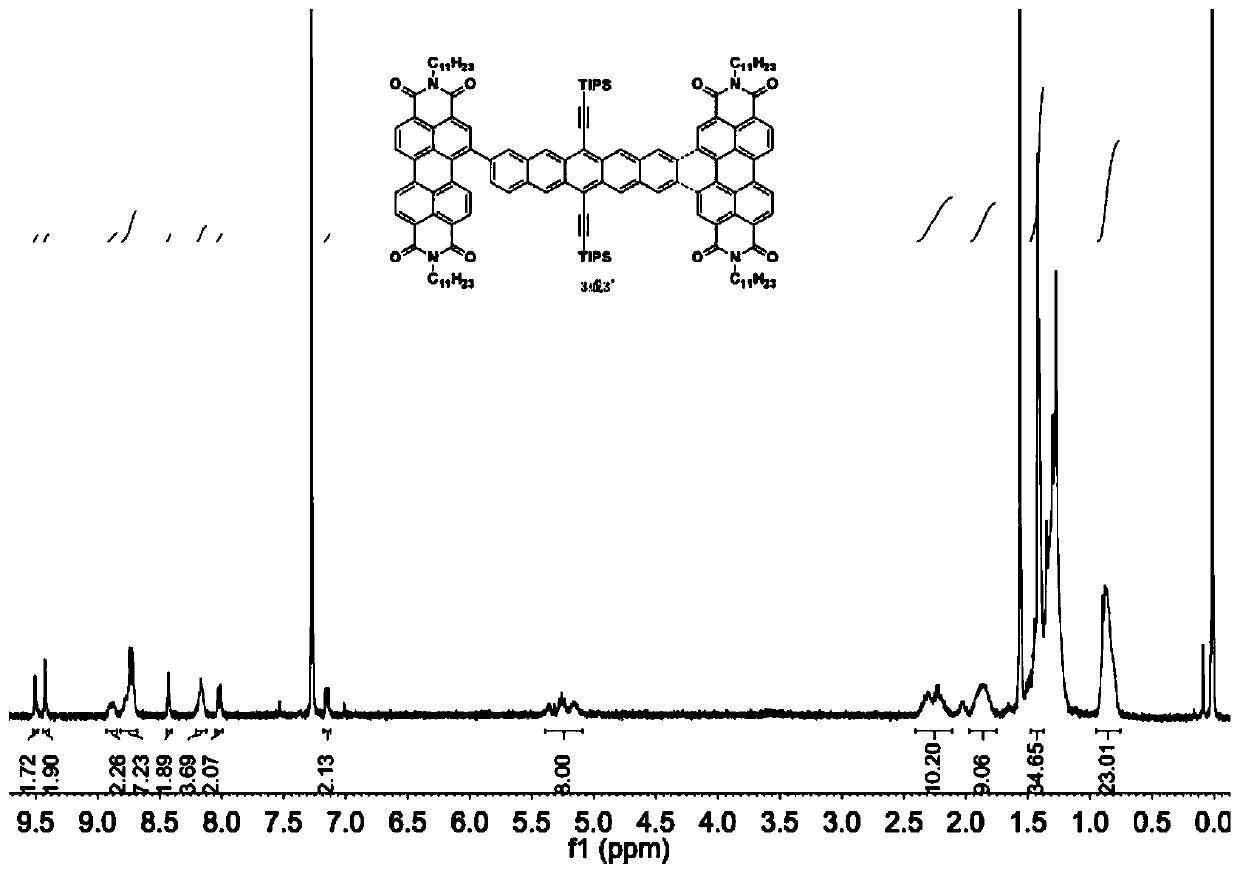
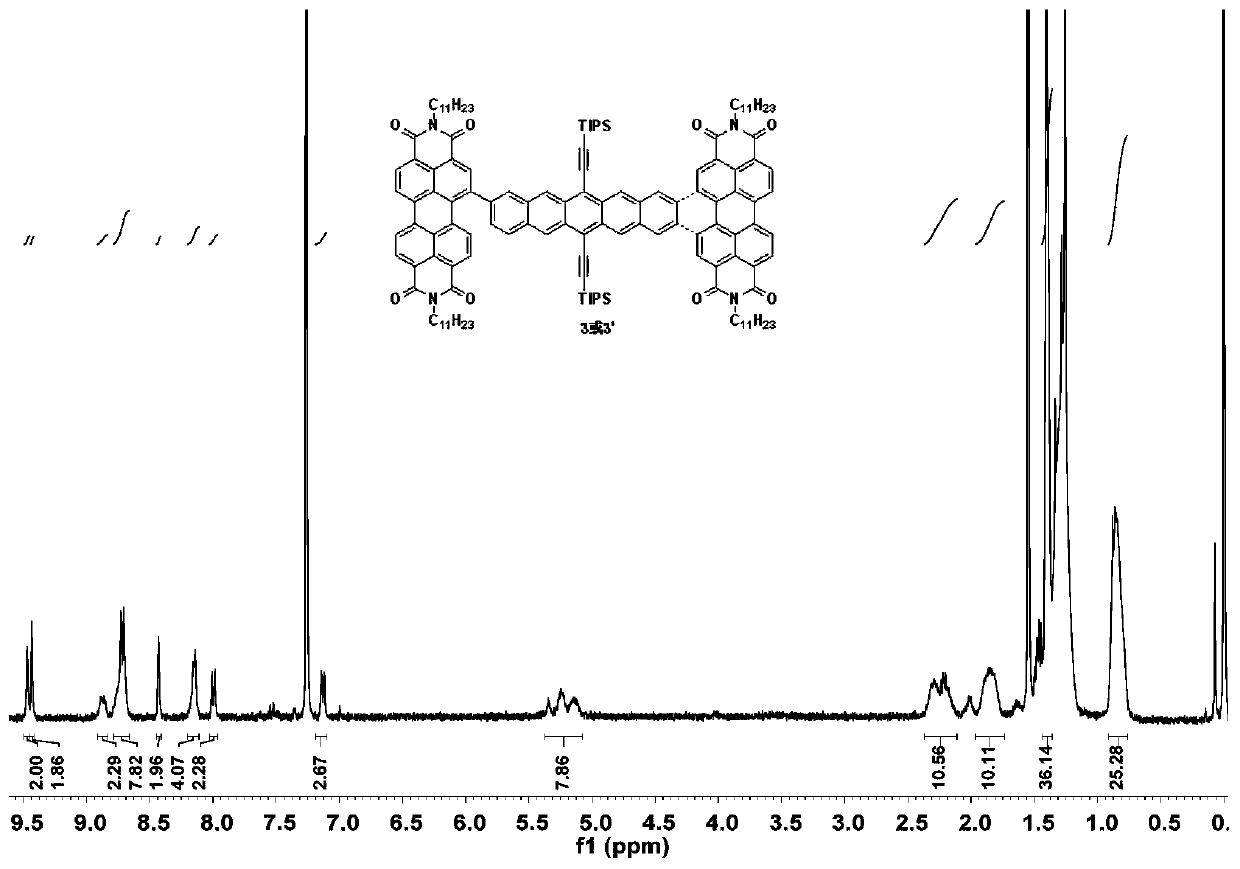
[0031] (1) Take substrates 1 (50mg, 0.056mmol) and 2 (95.67mg, 0.123mmol), dissolve them in a mixed solvent composed of 10mL toluene, 2mL ethanol, and 2mL water, and quickly add tetrakis(triphenylene) after nitrogen bubbles for 40min. Phosphine) palladium (6.47mg, 0.0056mmol) and potassium carbonate (46.44mg, 0.336mmol), and then blow nitrogen for 20min. Heated to 110°C and reacted for 24 hours. After the reaction was completed, concentrated and spin-dried, separated by column chromatography to obtain 3 and 3'.
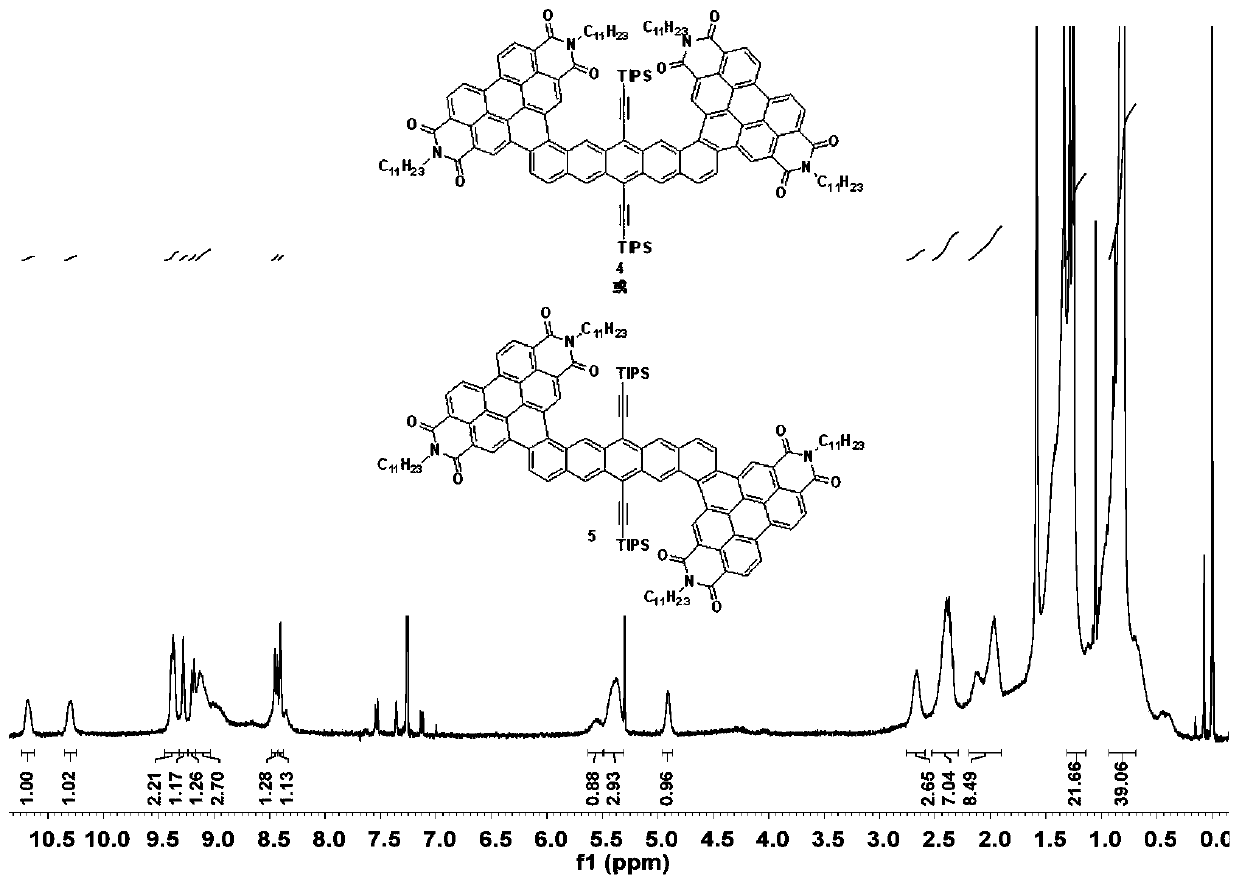
[0032] (2) Take substrate 3 (200.00mg, 0.0984mmol) and 3' (200.00mg, 0.0984mmol) respectively in two Schlenk tubes, add 50mL anhydrous and oxygen-free toluene solvent, iodine element (750mg, 2.94mmol) , using the method of freezing-pumping-thawing to remove oxygen, under the light condition of LED lamp (power 150W, luminous flux ...
Embodiment 2
[0034] The synthetic method of the A-D-A type organic optoelectronic small molecule based on perylene diimide adopts the following steps:
[0035] (1) Take substrates 1 (75mg, 0.084mmol) and 2 (156.81mg, 0.202mmol), dissolve them in a mixed solvent composed of 20mL toluene, 5mL ethanol, and 5mL water, and quickly add tetrakis(triphenylene) after nitrogen bubbles for 40min. Phosphine) palladium (7.765mg, 0.0067mmol) and potassium carbonate (58.00mg, 0.420mmol), and then blow nitrogen for 20min. Heated to 80°C and reacted for 12 hours. After the reaction, concentrated and spin-dried, separated by column chromatography to obtain 3 and 3'.
[0036](2) Take substrate 3 (100.00mg, 0.0492mmol) and 3' (100.00mg, 0.0492mmol) respectively in two Schlenk tubes, add 25mL anhydrous and oxygen-free toluene solvent, iodine simple substance (187.45mg, 0.738mmol ), using the method of freezing-pumping-thawing to remove oxygen, under the light condition of LED lamp (power 150W, luminous flux 1...
Embodiment 3
[0038] The synthetic method of the A-D-A type organic optoelectronic small molecule based on perylene diimide adopts the following steps:
[0039] (1) Take substrates 1 (150mg, 0.168mmol) and 2 (313.62mg, 0.404mmol), dissolve them in a mixed solvent composed of 40mL toluene, 8mL ethanol, and 8mL water, and quickly add tetrakis (triphenyl) after bubbling nitrogen gas for 40min. Phosphine) palladium (29.12mg, 0.0252mmol) and potassium carbonate (139.32mg, 1.008mmol), and then blow nitrogen for 20min. Heated to 140°C and reacted for 36 hours. After the reaction, concentrated and spin-dried, separated by column chromatography to obtain 3 and 3'.
[0040] (2) Take substrate 3 (50.00mg, 0.0246mmol) and 3' (100.00mg, 0.0246mmol) respectively in two Schlenk tubes, add 15mL anhydrous and oxygen-free toluene solvent, iodine element (200mg, 0.787mmol) , use the method of freezing-pumping-thawing to remove oxygen, and react for 40 hours under the light condition of LED lamp (power 150W, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color rendering index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com