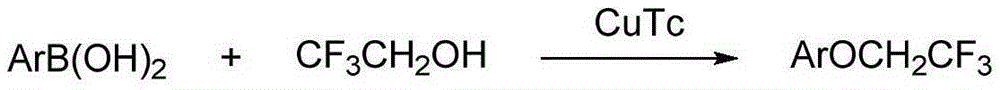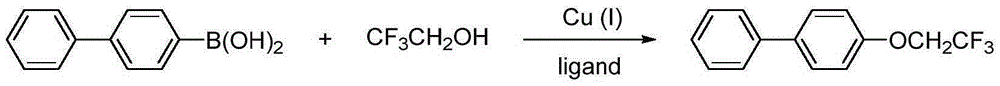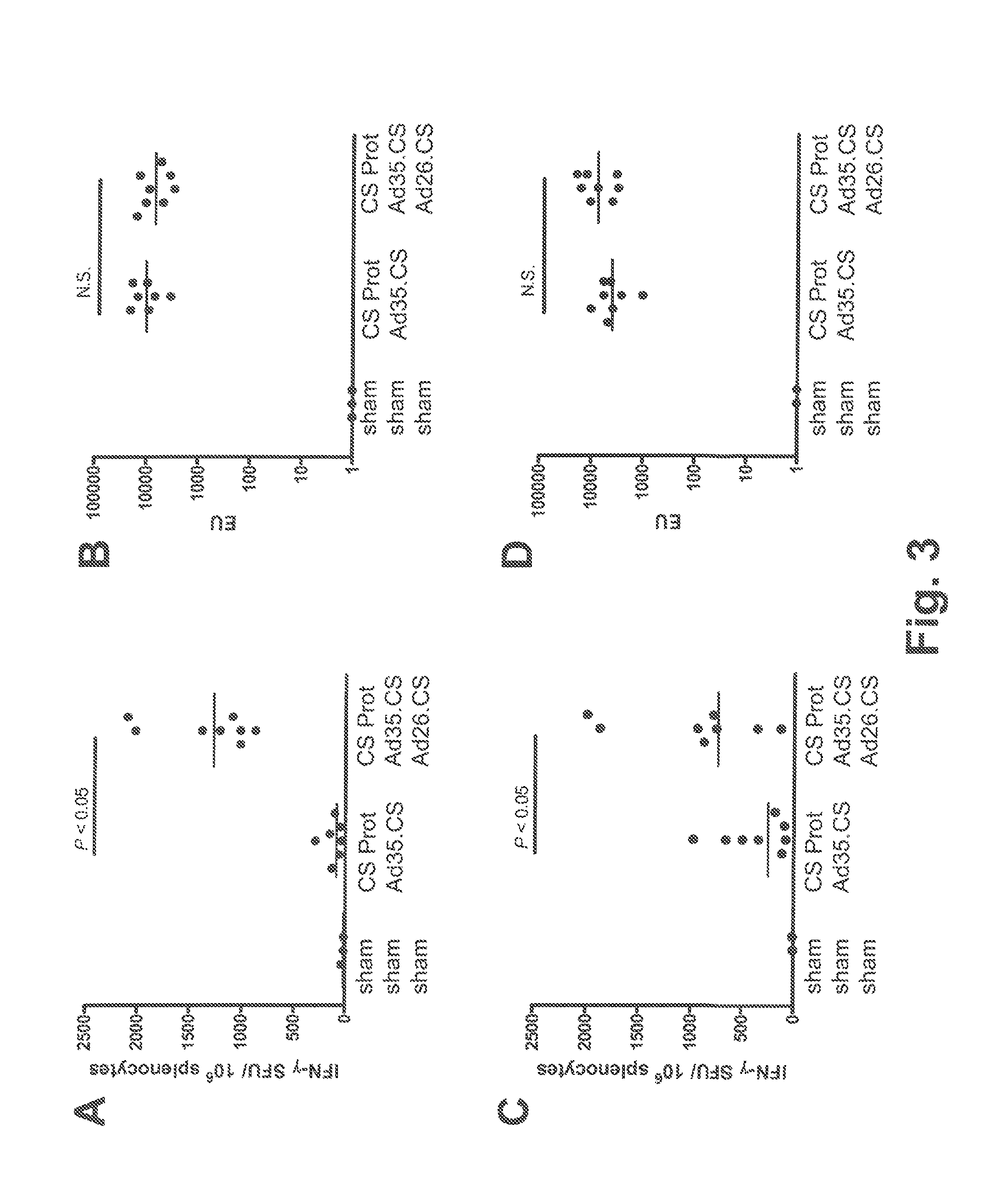Patents
Literature
31results about How to "Poor response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
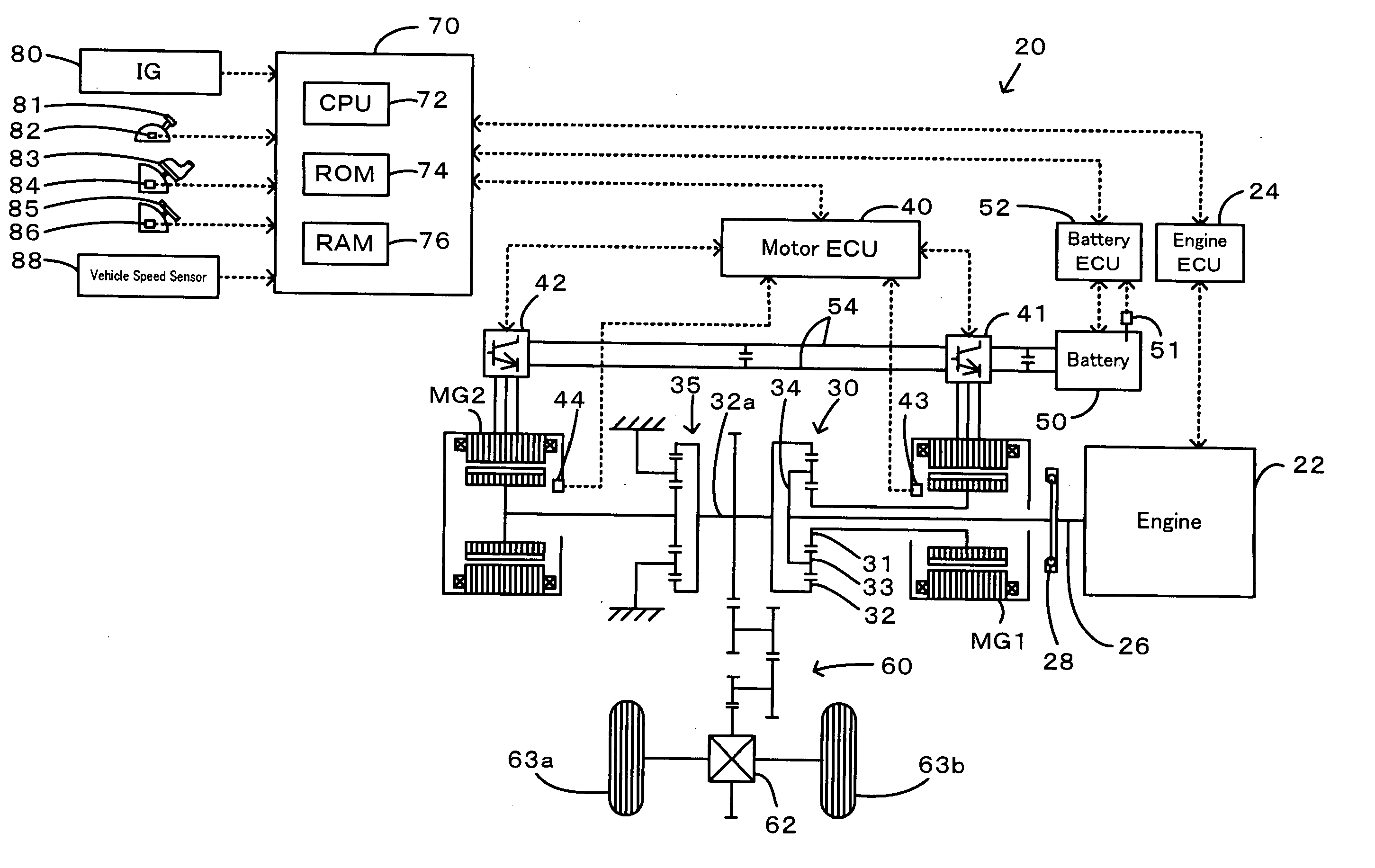
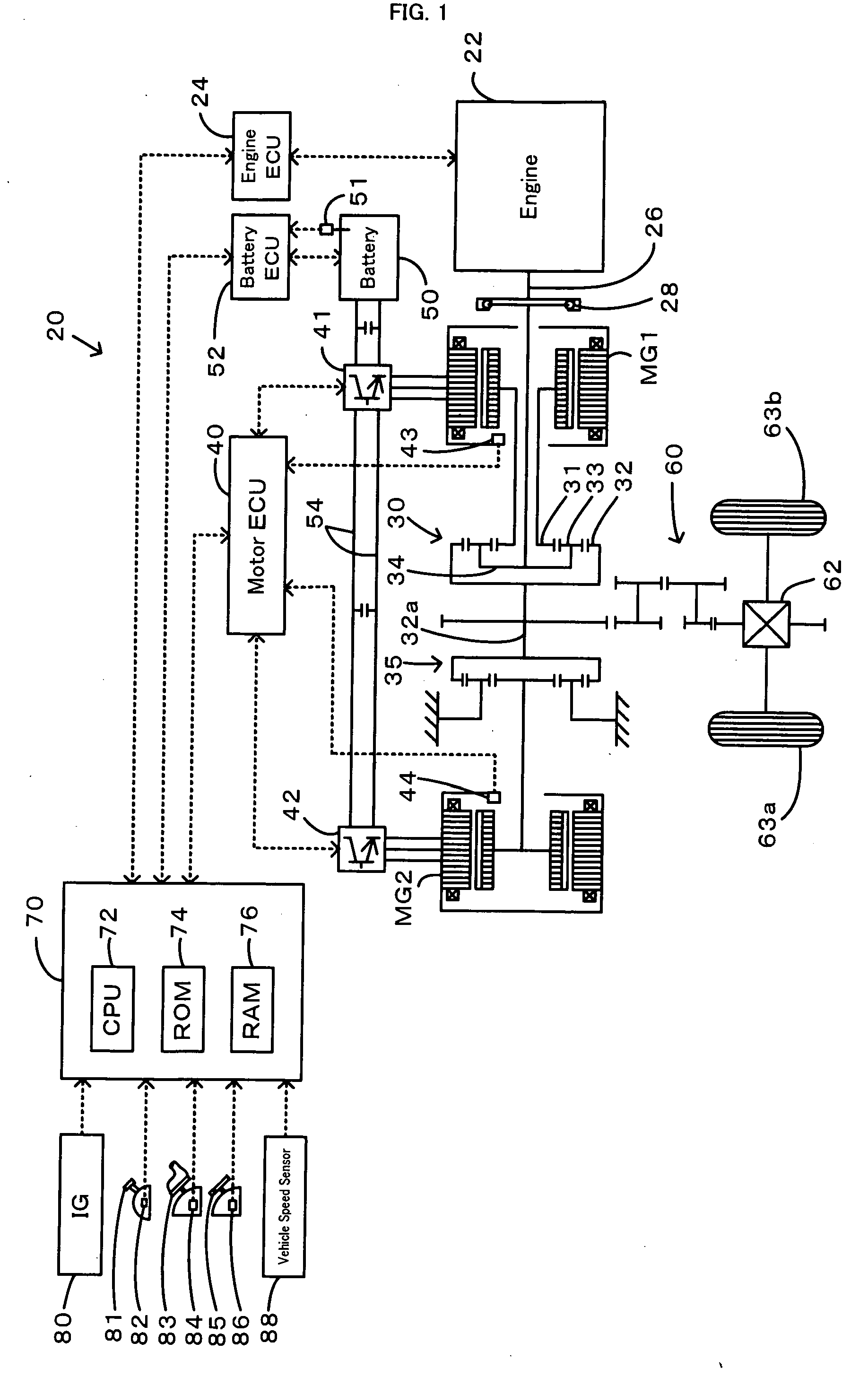
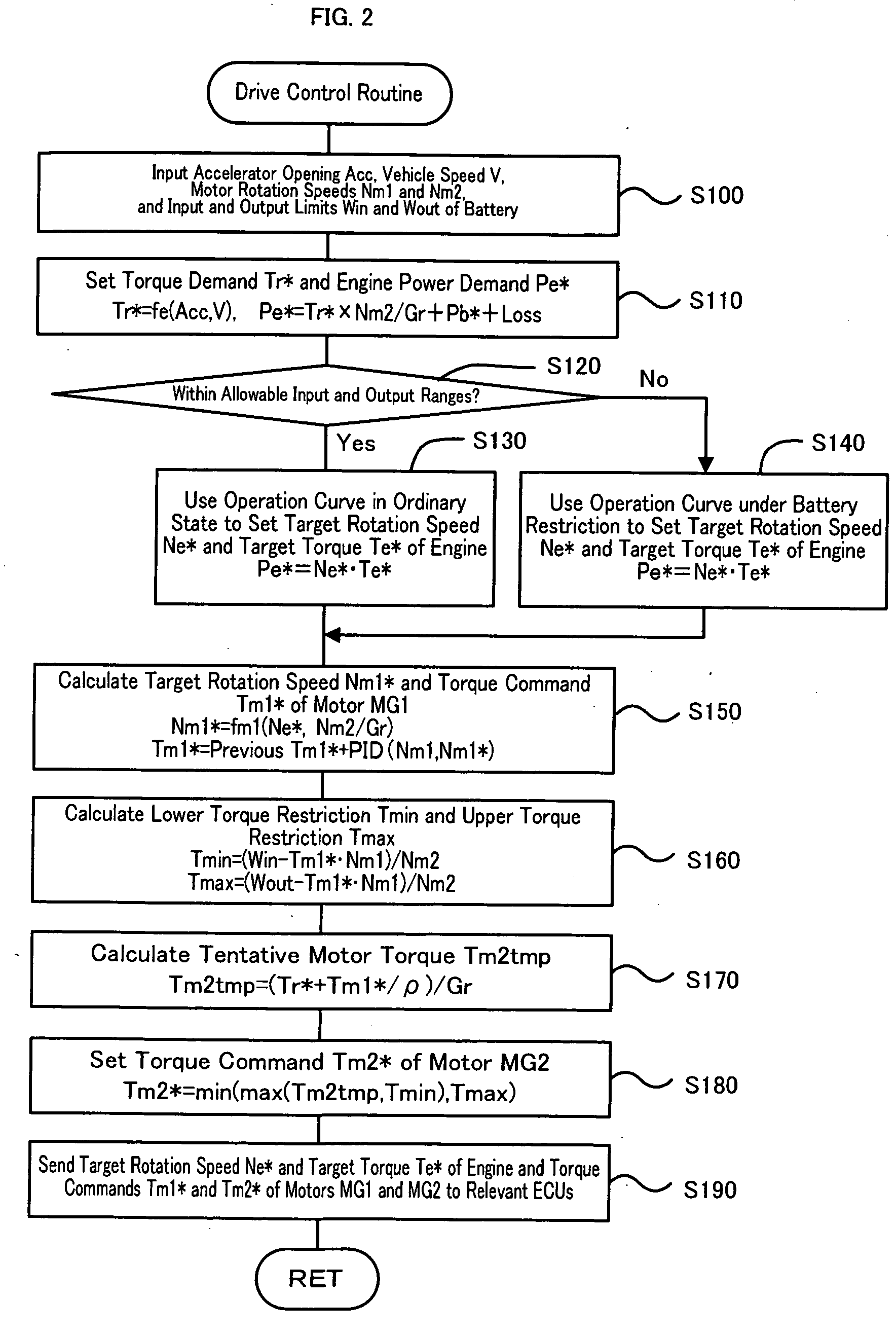
Power Output Apparatus, Motor Vehicle Equipped with Power Output Apparatus, and Control Method of Power Output Apparatus
InactiveUS20070255463A1Poor responseInsufficient powerElectrical controlDigital data processing detailsMobile vehicleDelayed response
When the current input and output charge levels of a battery are out of allowable input and output ranges, the drive control of the invention uses an operation curve under battery restriction, which sets the higher rotation speed in a low power range and has a smaller variation in rotation speed against a variation of output power than an operation curve in the ordinary state, to set a target rotation speed Ne* and a target torque Te* of an engine and controls the engine and motors MG1 and MG2. Application of this operation curve under battery restriction enhances the response of the engine to a variation of engine power demand Pe* and decreases a potential insufficiency of power due to a delayed response of the engine. The battery can thus input and output a required electric power within the ranges of an input limit Win and an output limit Wout to ensure supply of the insufficient power from the motor MG2. This control enables smooth output of a required power to a driveshaft.
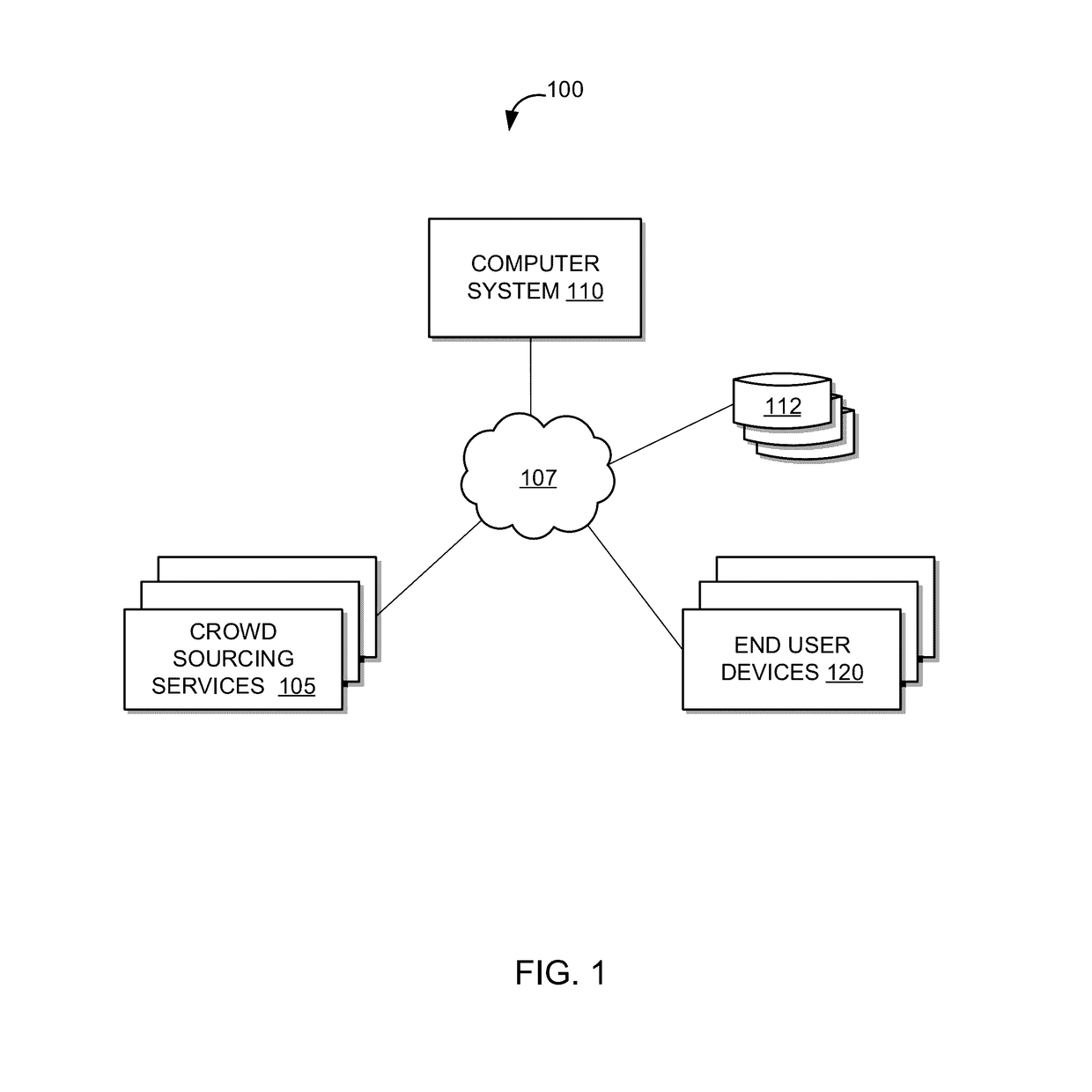
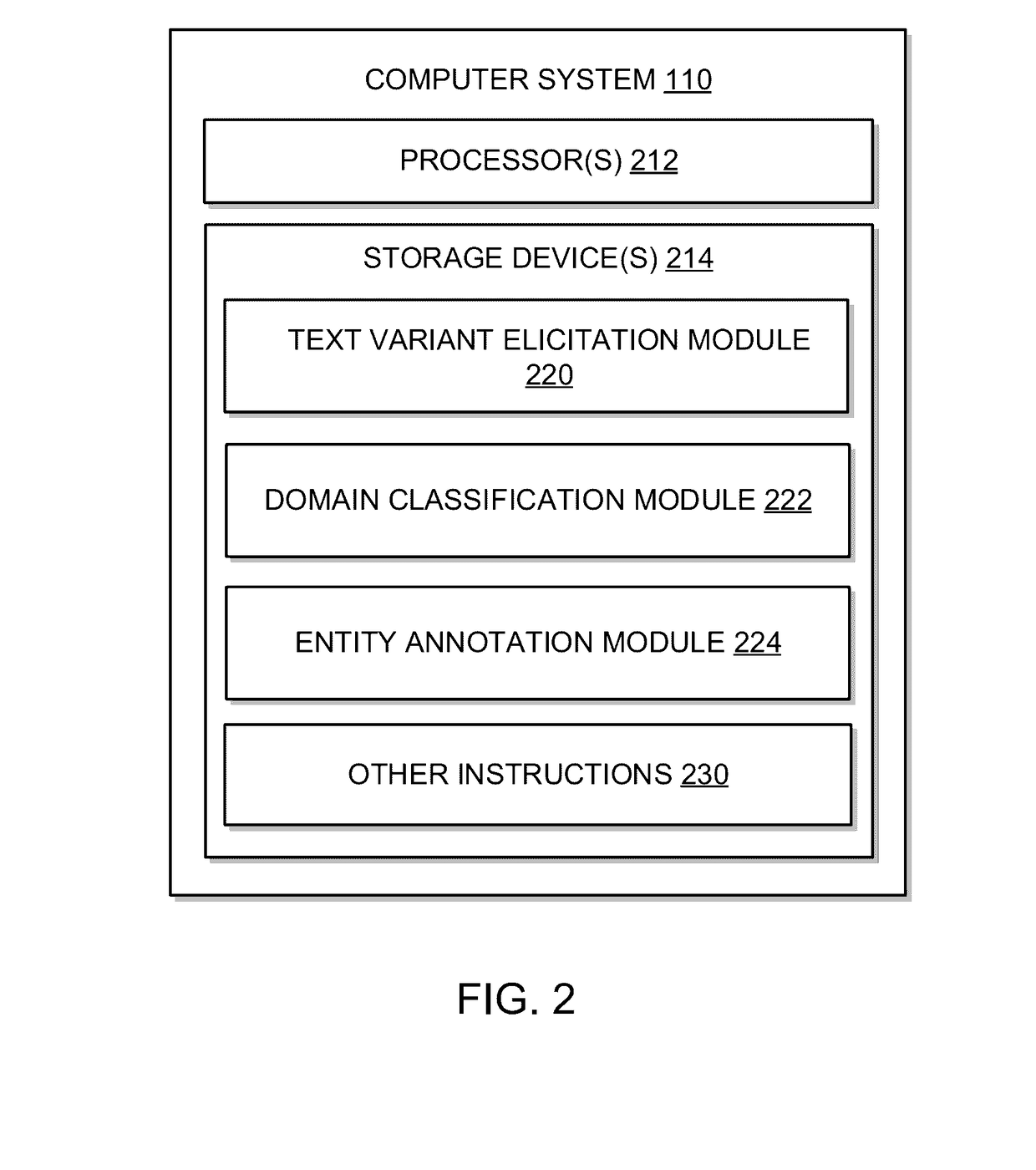
System and method of annotating utterances based on tags assigned by unmanaged crowds
ActiveUS20170068651A1Increase in sizeReduce cognitive loadSemantic analysisSpecial data processing applicationsNamed-entity recognitionCrowds
A system and method of tagging utterances with Named Entity Recognition (“NER”) labels using unmanaged crowds is provided. The system may generate various annotation jobs in which a user, among a crowd, is asked to tag which parts of an utterance, if any, relate to various entities associated with a domain. For a given domain that is associated with a number of entities that exceeds a threshold N value, multiple batches of jobs (each batch having jobs that have a limited number of entities for tagging) may be used to tag a given utterance from that domain. This reduces the cognitive load imposed on a user, and prevents the user from having to tag more than N entities. As such, a domain with a large number of entities may be tagged efficiently by crowd participants without overloading each crowd participant with too many entities to tag.
Owner:VOICEBOX TECH INC
Method of Treating Cancer Using Dithiocarbamate Derivatives
InactiveUS20070232692A1Readily available and easily used treatmentEffective treatmentHeavy metal active ingredientsBiocideThiocarbamateAnticarcinogen
Dithiocarbamate, particularly tetraethylthiuram disulfide, and thiocarbamate anions thereof, strongly inhibit the growth of cancer cells of a variety of cell types. Such inhibitory effect is enhanced by heavy metal ions such as copper ions, cytokines and ceruloplasmin. A method is presented for using tetraethylthiuram disulfide to reduce tumor growth, and to potentiate the effect of other anticancer agents.
Owner:THE UNIV OF UTAH
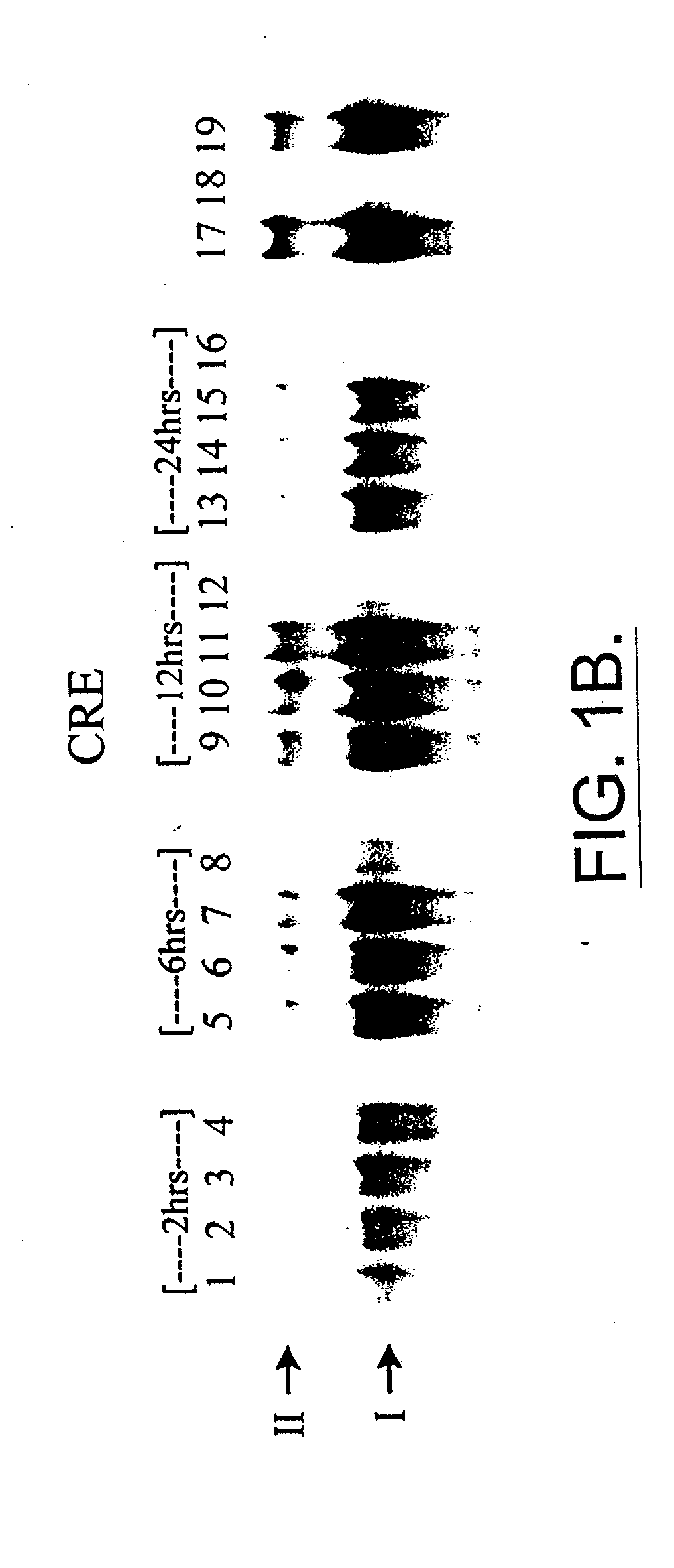
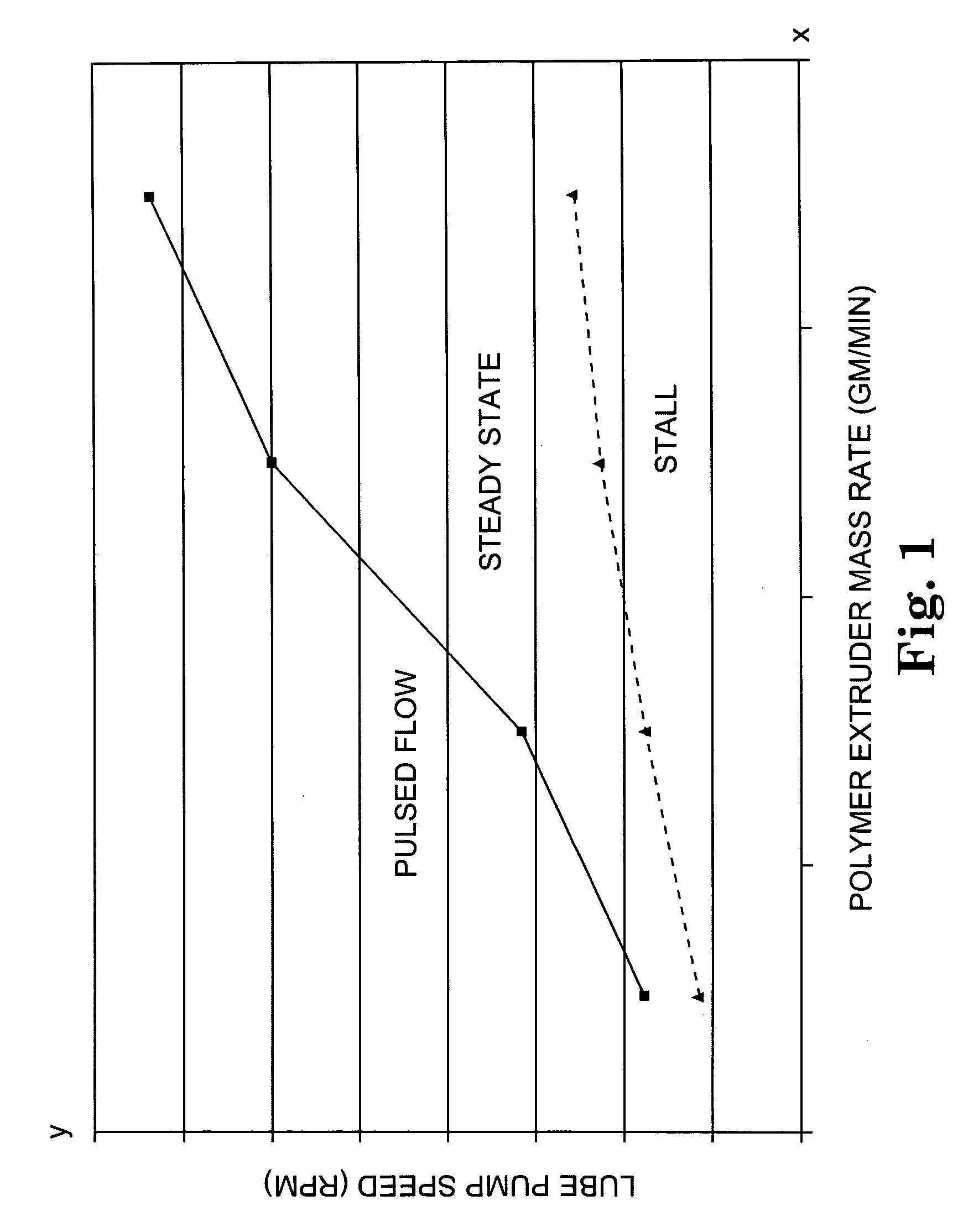
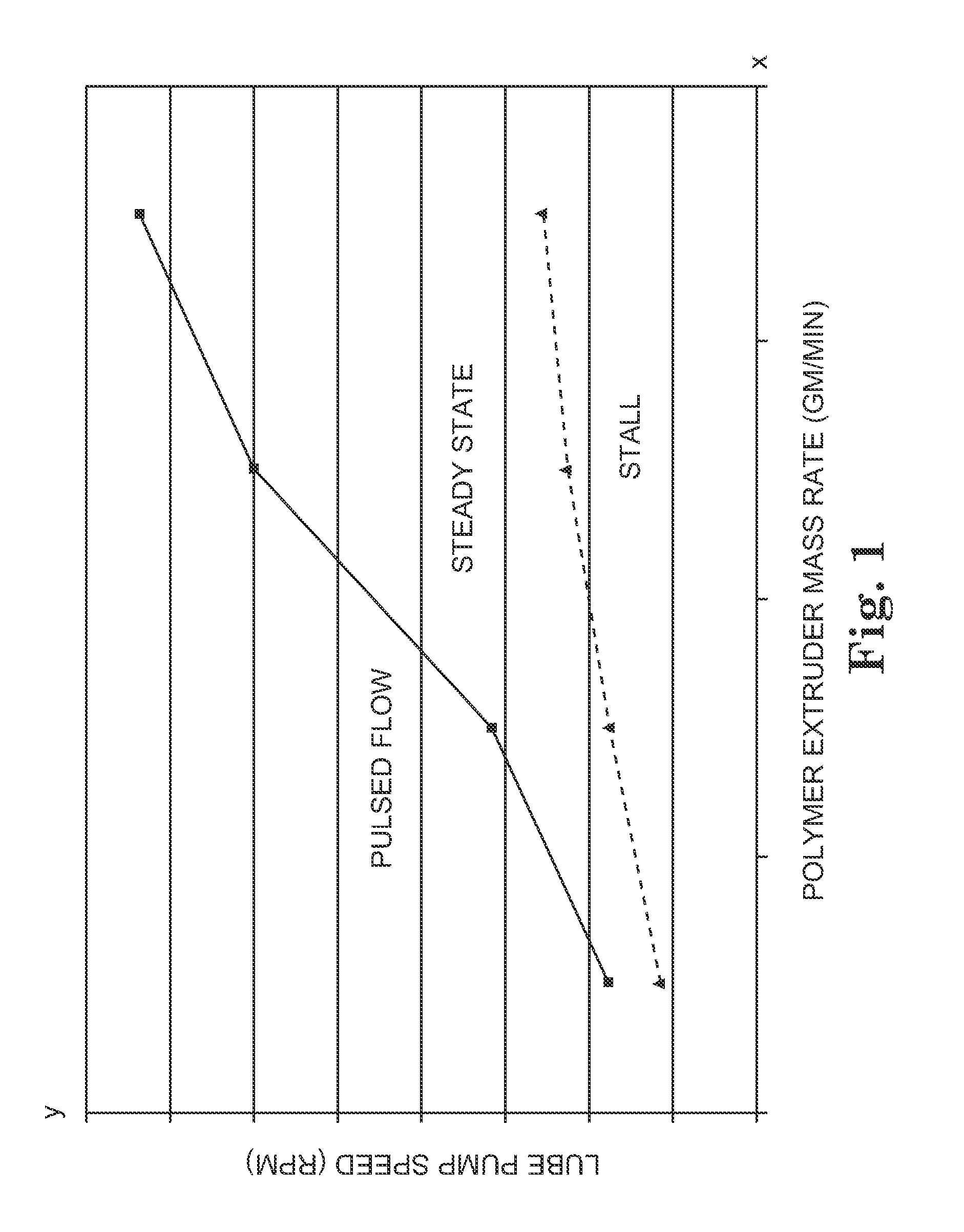
Lubricated flow fiber extrusion
InactiveUS20050258562A1Melt viscosityPoor responseSpinnerette packsSpinning head liquid feederFiberPolymer science
Methods and systems for extruding polymeric fibers are disclosed. The extrusion process preferably involves the delivery of a lubricant separately from a polymer melt stream to each orifice of an extrusion die such that the lubricant preferably encases the polymer melt stream as it passes through the die orifice.
Owner:3M INNOVATIVE PROPERTIES CO
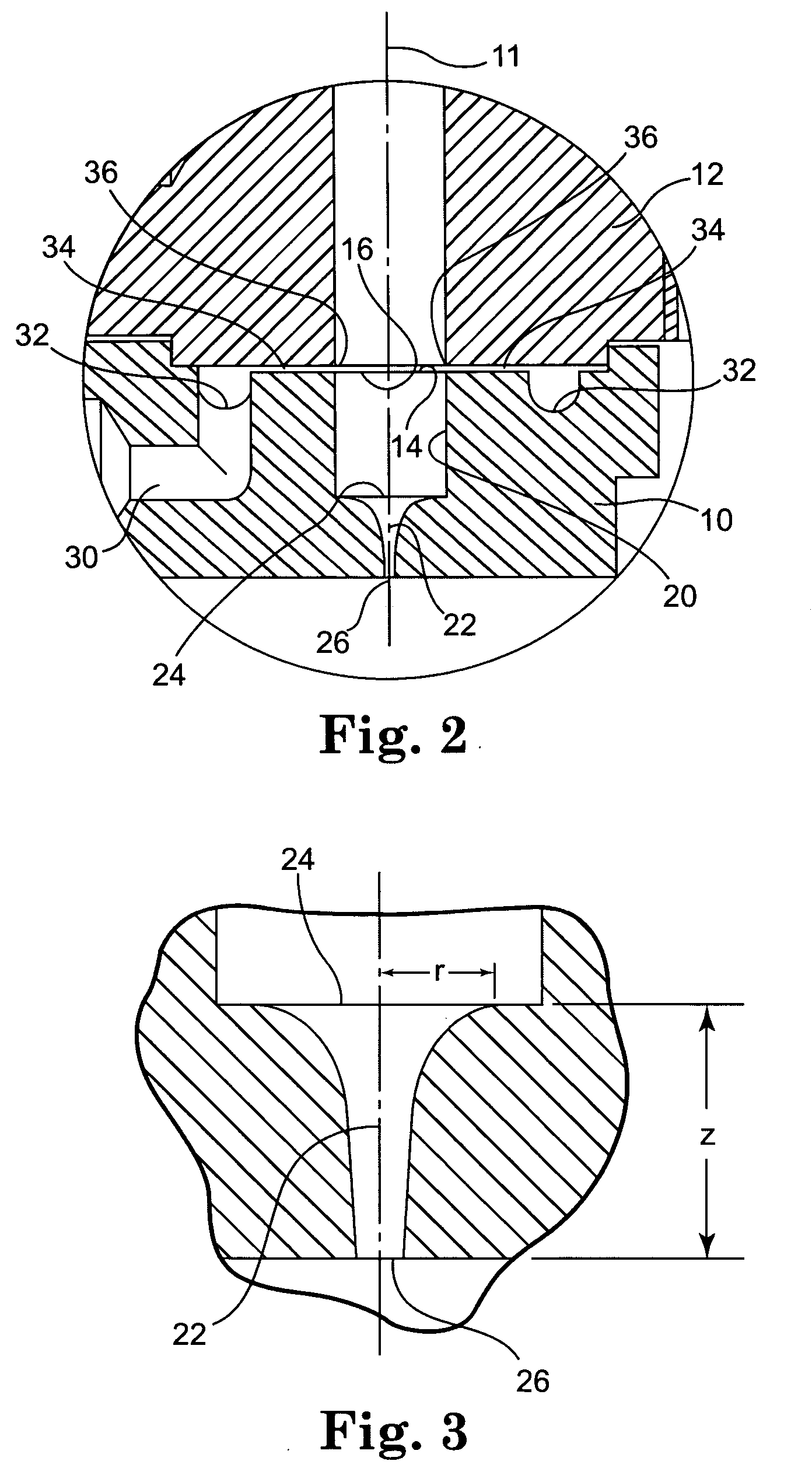
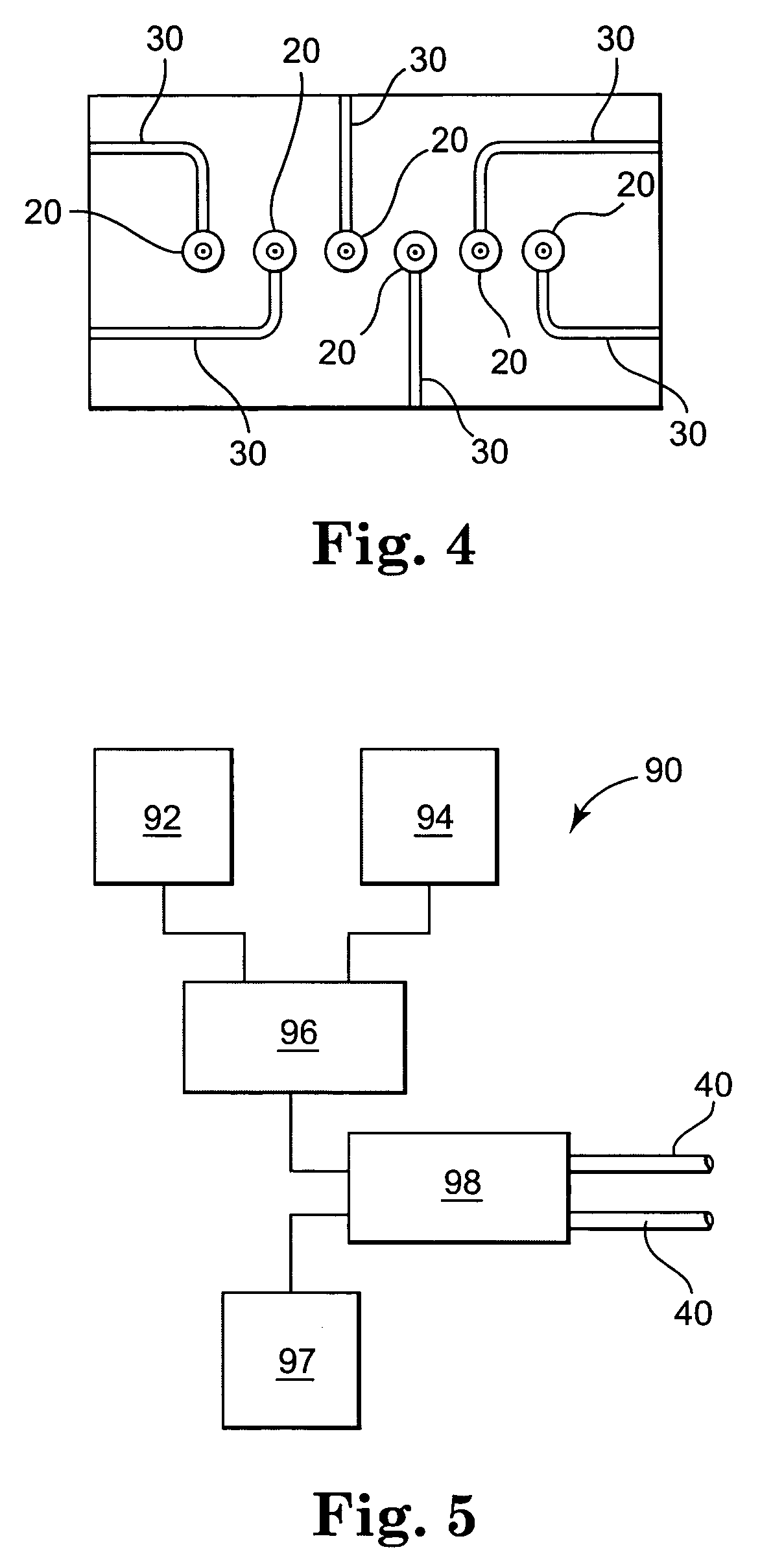
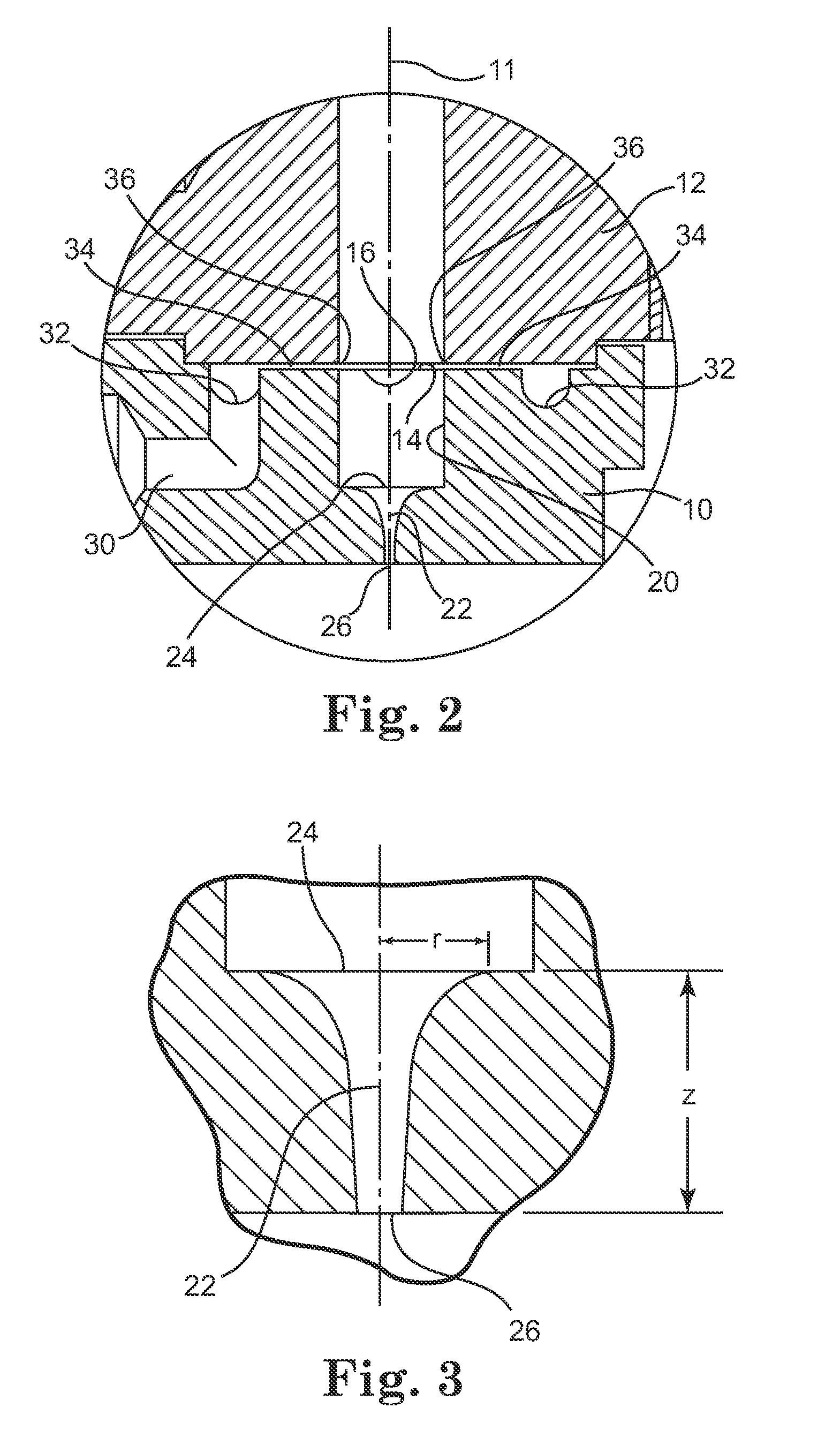
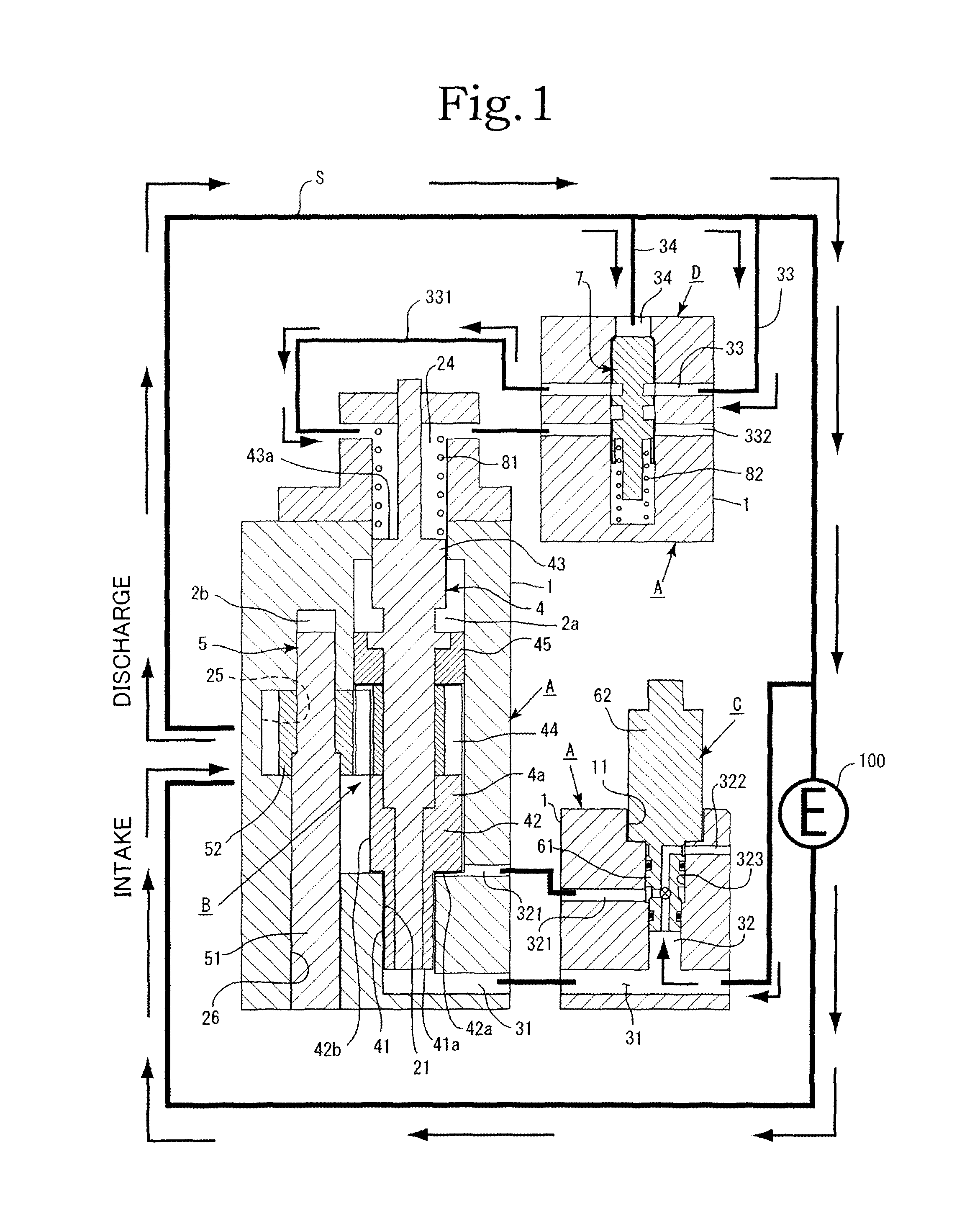
Pump device
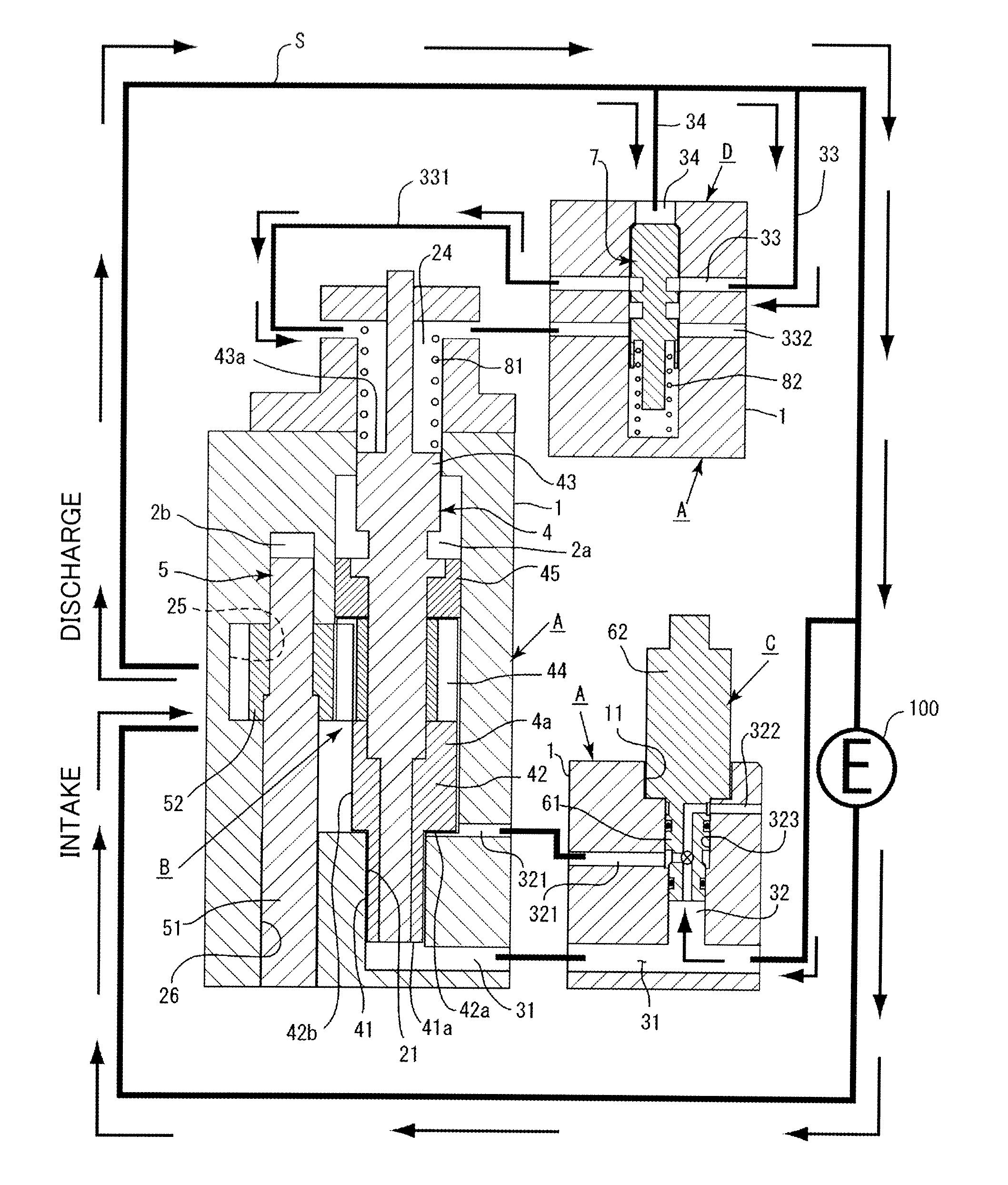
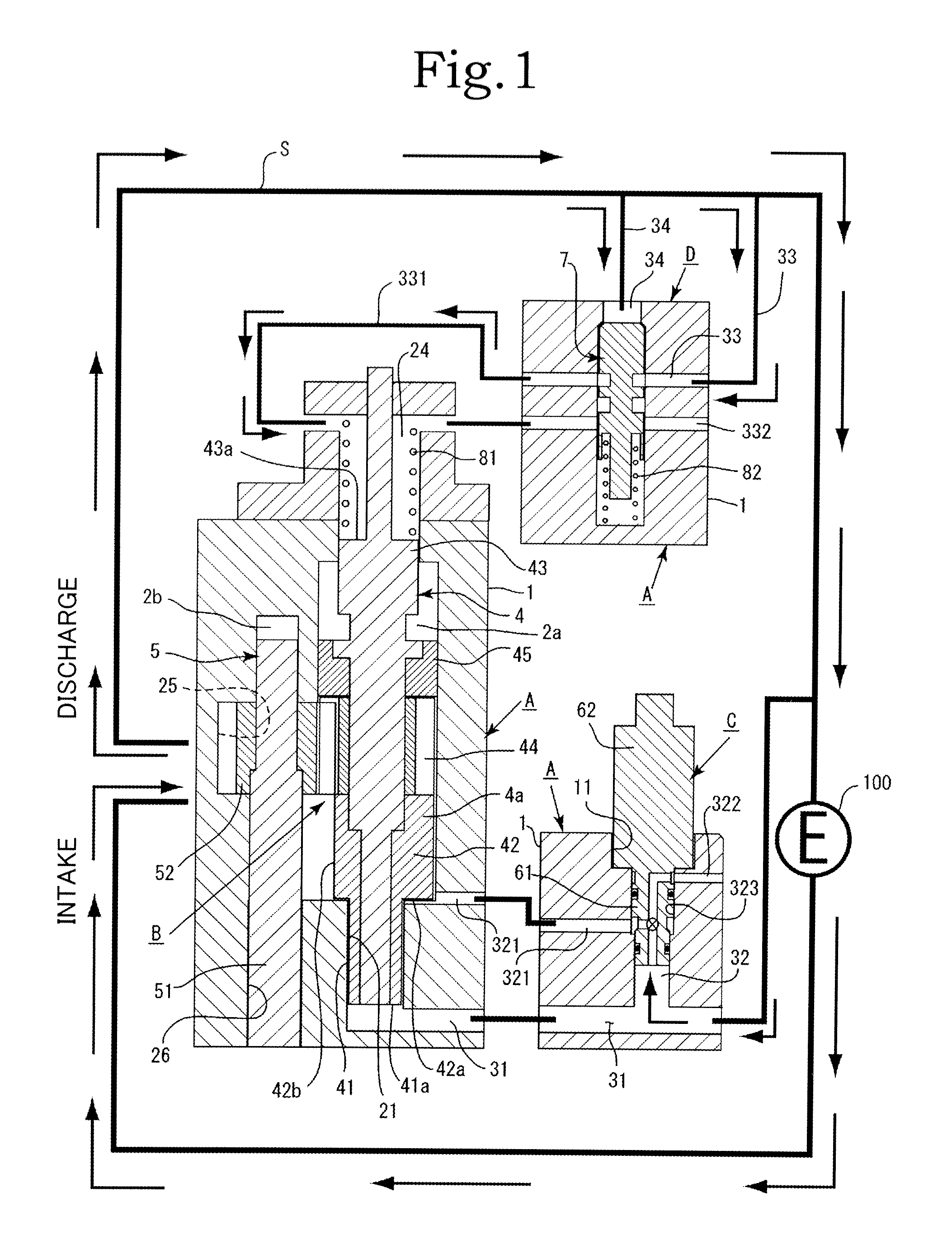
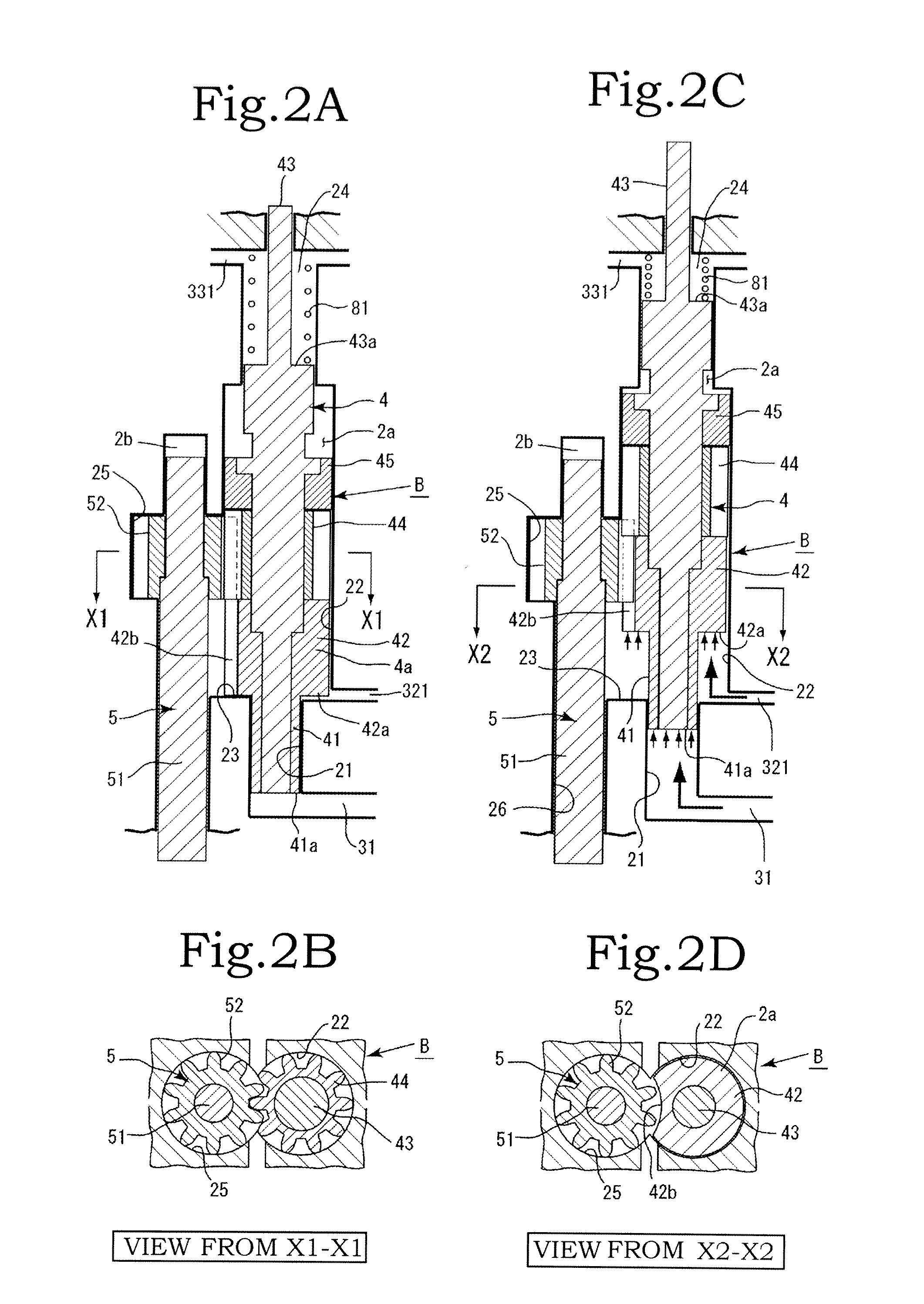
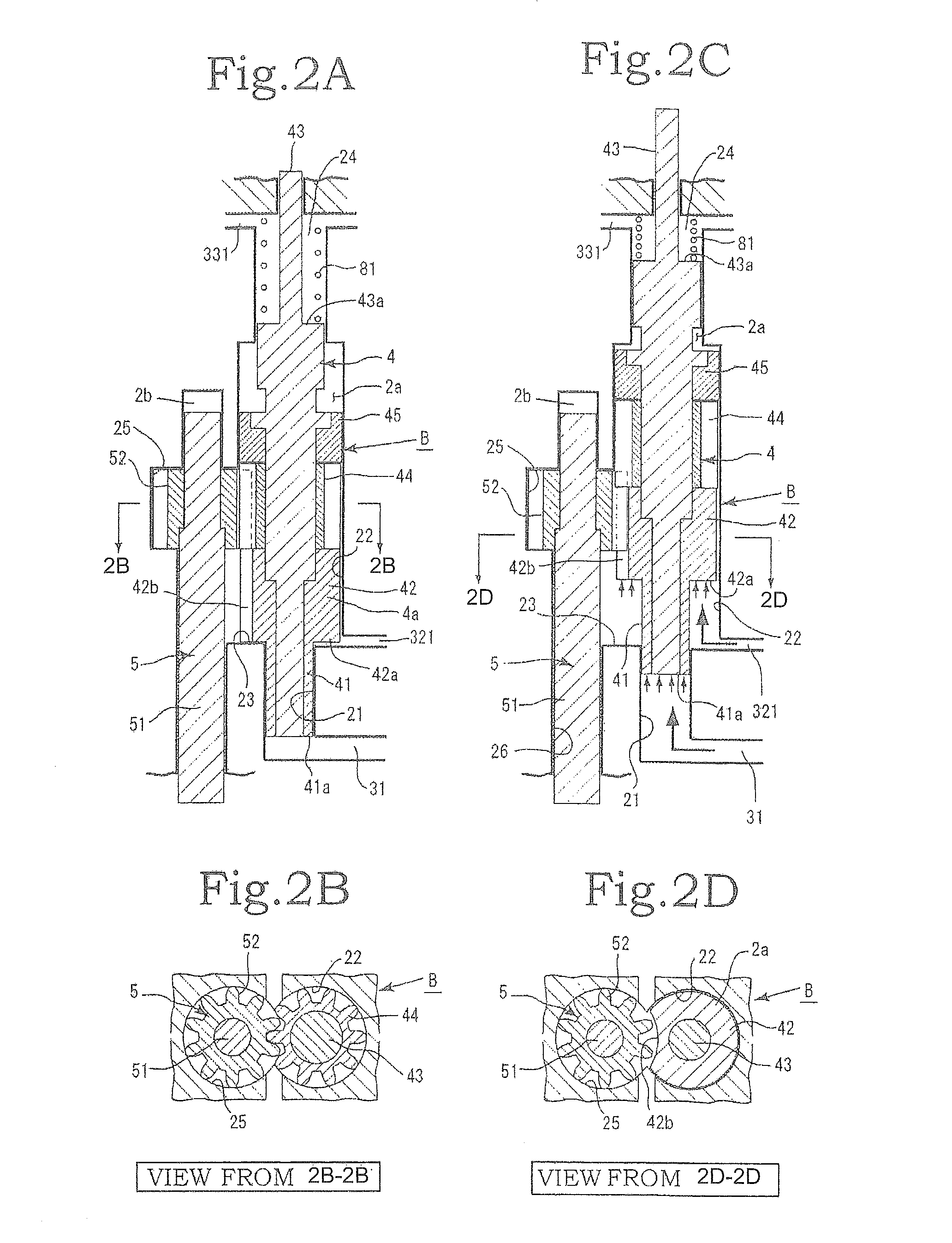
InactiveUS20120244027A1Discharge volume can be quicklyRapid reductionOscillating piston enginesEngine of intermeshing engagement typeVolume reductionOil pressure
The invention has: a housing; a pump section formed of a drive gear unit and a driven gear unit; a main flow channel through which oil pressure is applied to the driven gear unit in a discharge volume reduction direction; a first branching flow channel through which oil pressure that assists oil pressure from the main flow channel is applied; a second branching flow channel through which oil pressure is applied to the driven gear unit in a discharge increase direction; a first flow channel control section; a second flow channel control section; and a spring that elastically urges the driven gear unit in a discharge increase direction. The first flow channel control section and the second flow channel control section can perform switching control in accordance with each increase or decrease of engine revolutions and in pressure.
Owner:YAMADA SEISAKUSHO KK
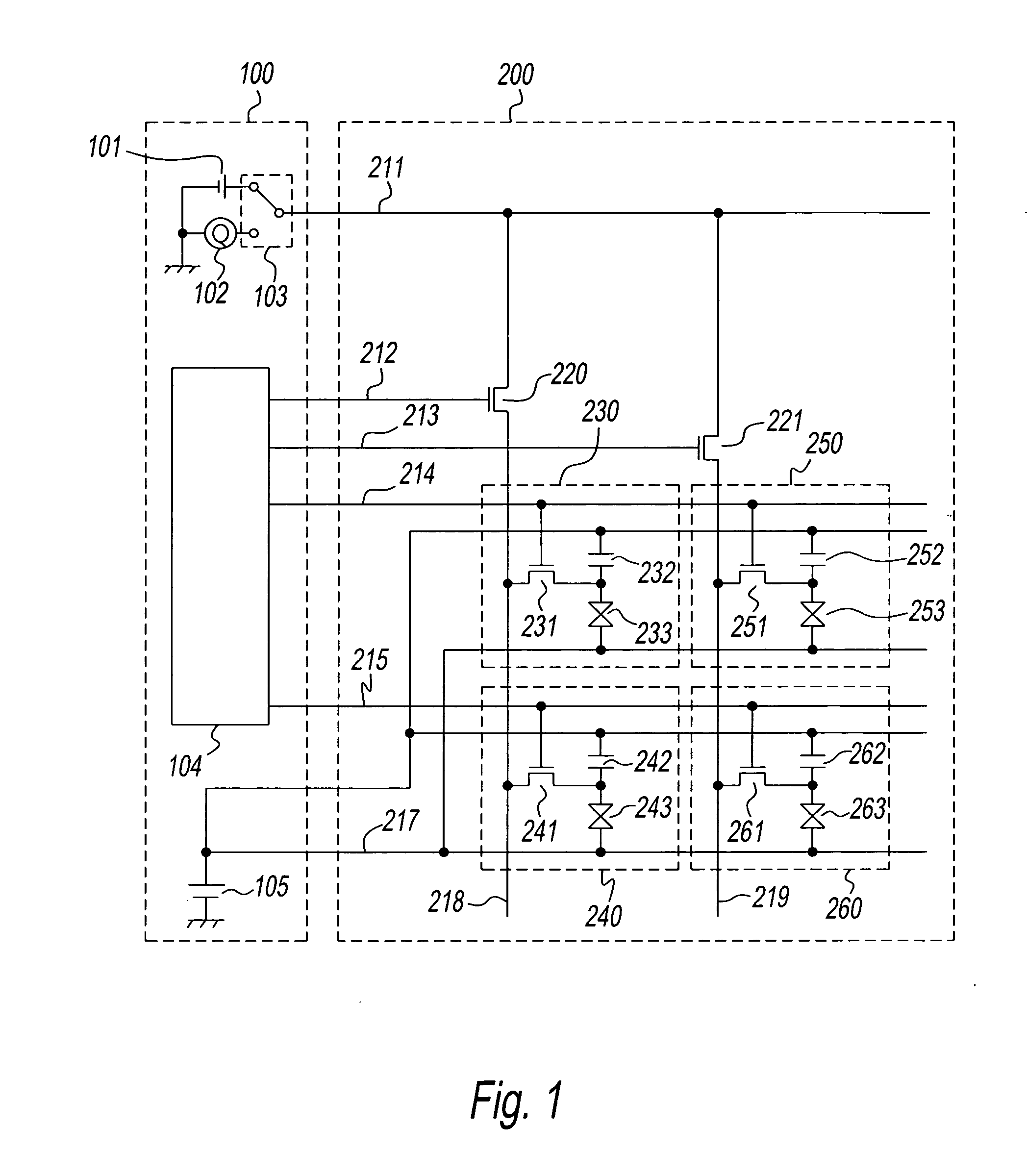
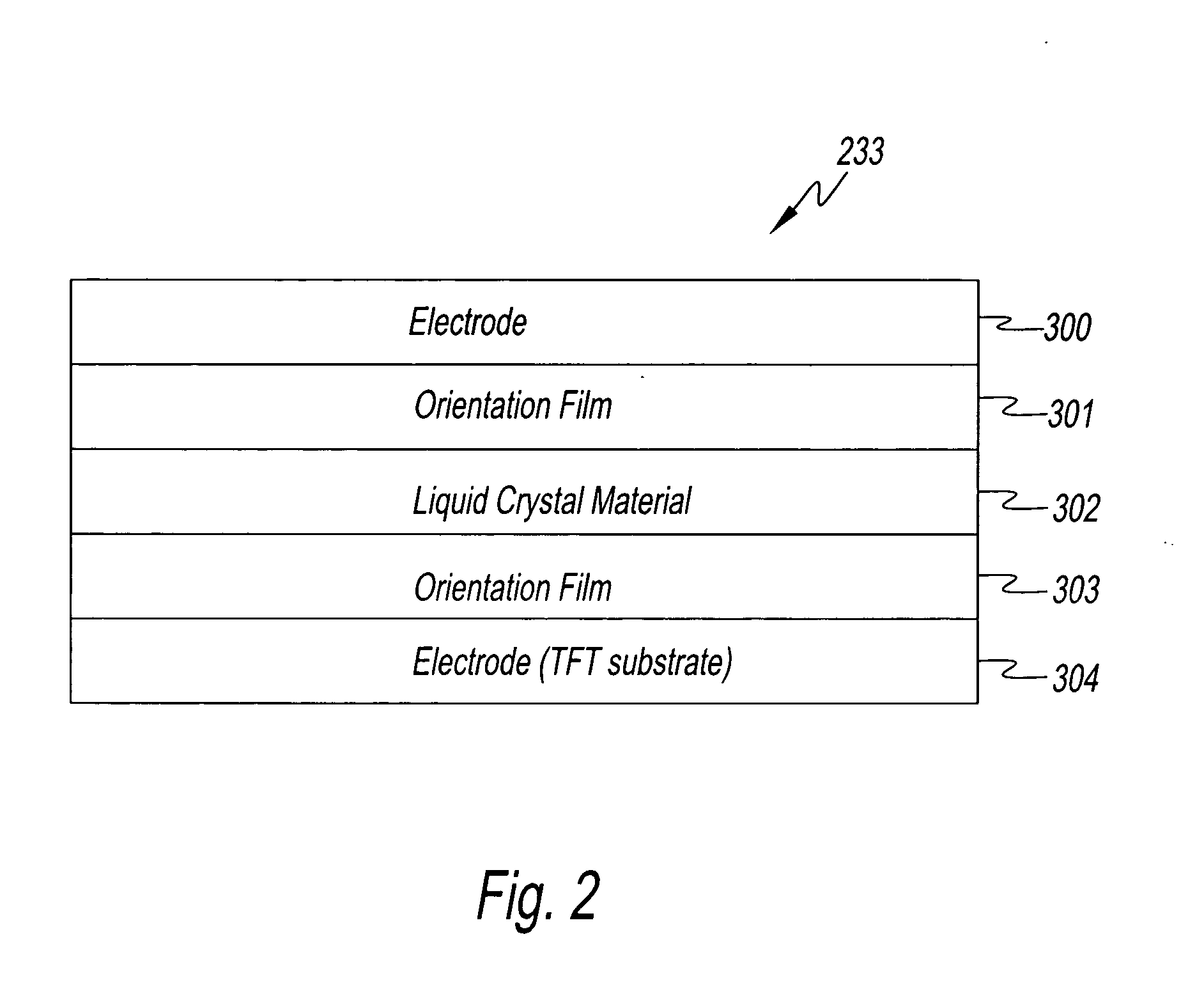
Method and apparatus for testing a TFT array for a liquid crystal panel
InactiveUS20070176623A1Accurate checkDetect defective pixelStatic indicating devicesIndividual semiconductor device testingLiquid-crystal displayElectrical polarity
A method for testing a liquid crystal display panel wherein there are, disposed in matrix form, pixels comprising liquid crystal elements in which a liquid crystal material is sealed between opposing electrodes, this method for testing a liquid crystal display panel being characterized in that it comprises a first charging step for applying a first voltage between the opposing electrodes of the liquid crystal element of the pixel under test; a second charging step for applying a second voltage of the opposite polarity of the first voltage between the opposing electrodes of the liquid crystal element of the pixel under test; and a measuring step for discharging the charge that has accumulated in the electrodes of the liquid crystal element of the pixel under test after the second charging step, and measuring the amount of charge discharged.
Owner:AGILENT TECH INC
Signal processing apparatus, signal processing method, and program
InactiveUS20100030917A1Excellent responsePoor responseTelevision system detailsColor television detailsEngineeringSignal processing
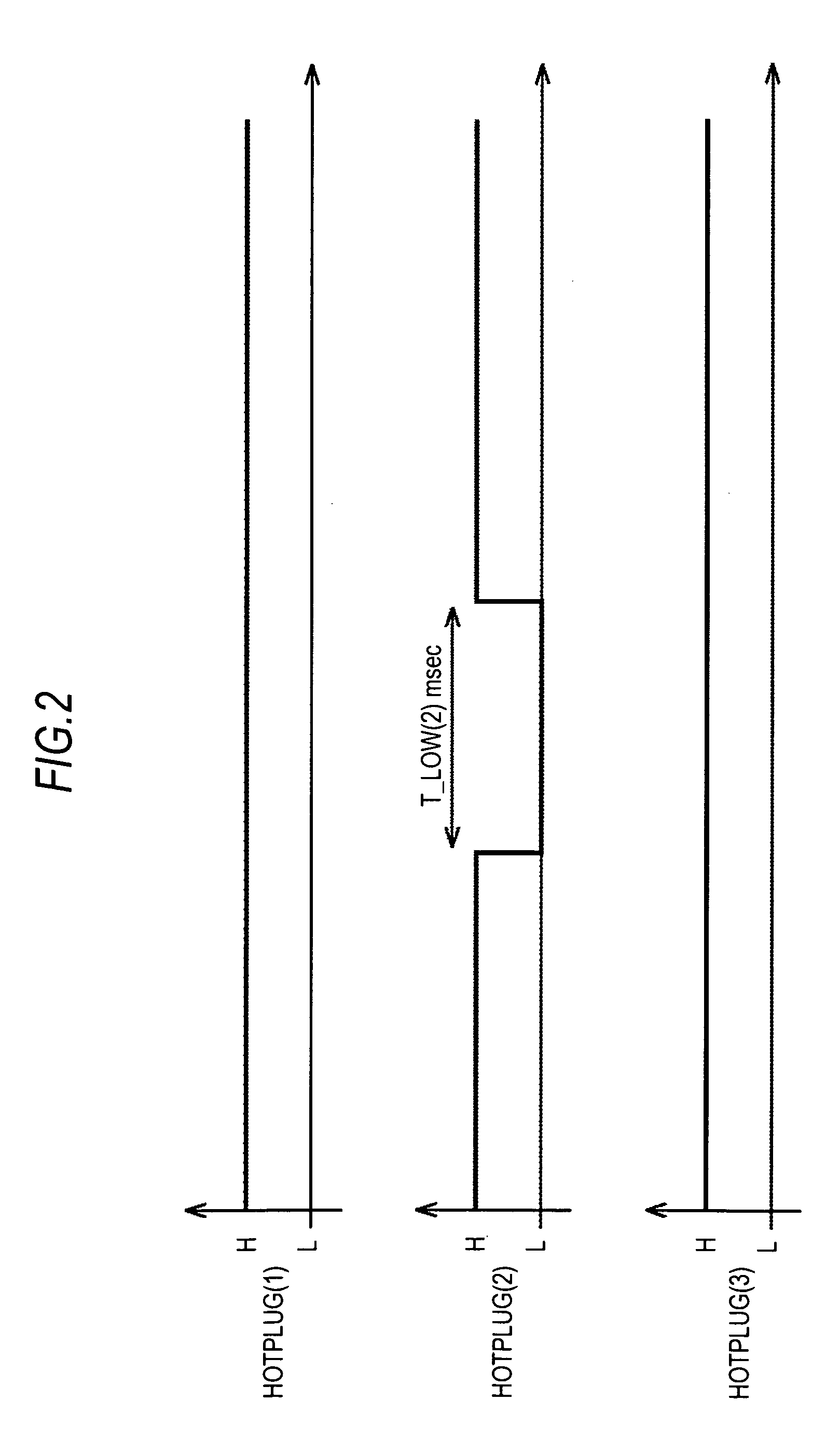
A signal processing apparatus includes: a connecting means for use in connecting to a different device; a signal control means for changing a control signal to be outputted to the different device through the connecting means for a predetermined period; a changing means for changing the predetermined period; a determining means for determining for each of the predetermined periods changed by the changing means whether the different device stably makes a response to a change in the control signal caused by the signal control means; and a deciding means for deciding a shortest predetermined period from the predetermined periods determined by the determining means that the different device stably makes a response, as a standby time for the different device connected through the connecting means.
Owner:SONY CORP
Method for preparing aryl trifluoroethoxyl ether
InactiveCN105348048AHigh reactivityMild conditionsCarbamic acid derivatives preparationCarboxylic acid nitrile preparationArylOrganic solvent
The invention relates to a method for preparing aryl trifluoroethoxyl ether. The method includes the steps that aryl boron compounds and trifluoroethanol are added to organic solvent, a copper salt catalyst, a ligand and an oxidizing agent are added, a reaction is conducted in a stirring mode for 1-40 hours at the temperature of 0-60 DEG C, filtering is conducted, column chromatography isolation is conducted, and the aryl trifluoroethoxyl ether is obtained. The method is simple in operation, raw materials are easy to obtain, the reaction condition is mild, the substrate universality is wide, the environmental friendliness is achieved, and the method is applicable to industrial application.
Owner:DONGHUA UNIV +1
Heterologous prime boost vaccination regimen against malaria
ActiveUS9168292B2High protection levelHigh levelOperating means/releasing devices for valvesProtozoa antigen ingredientsAntigenRegimen
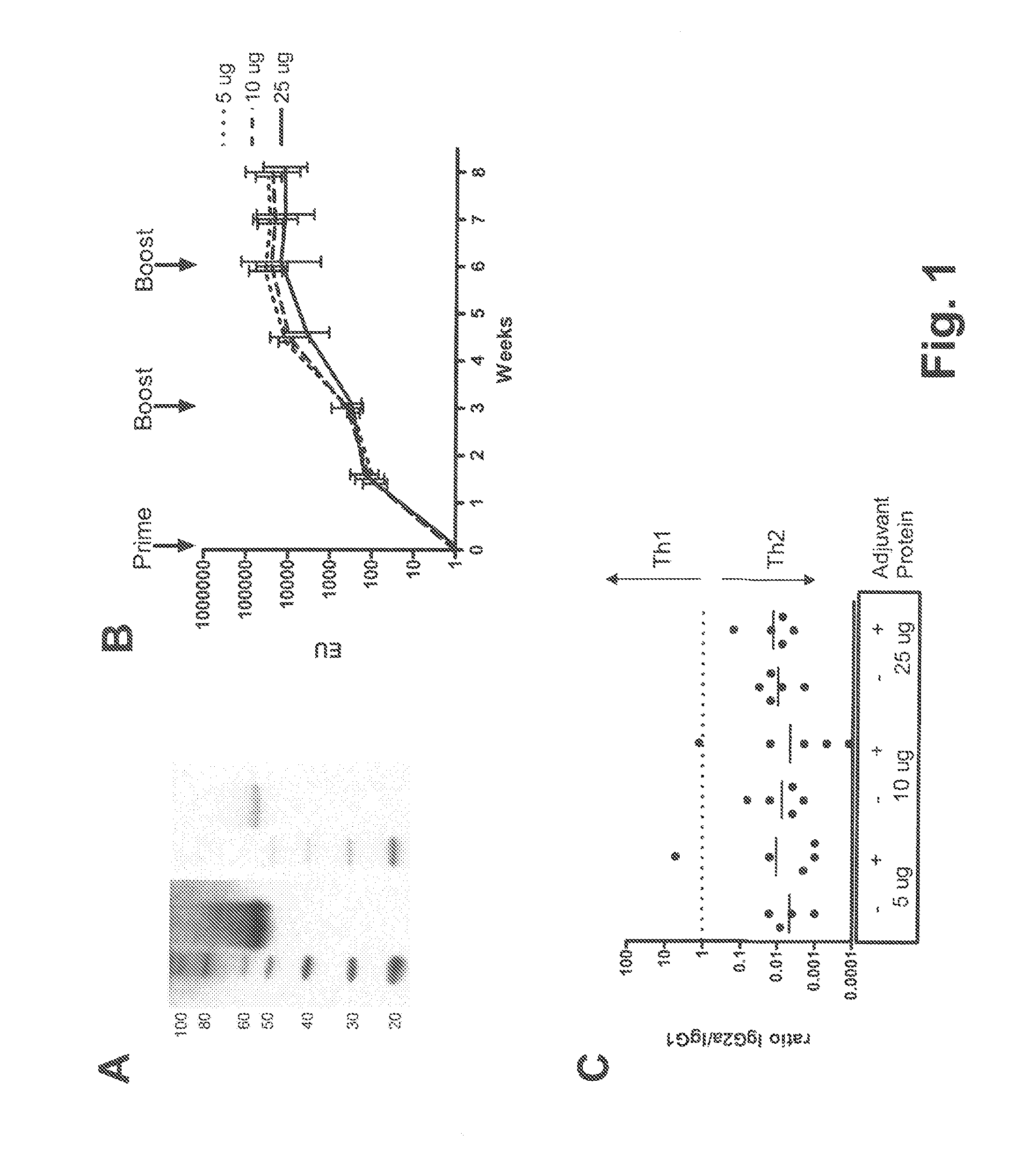
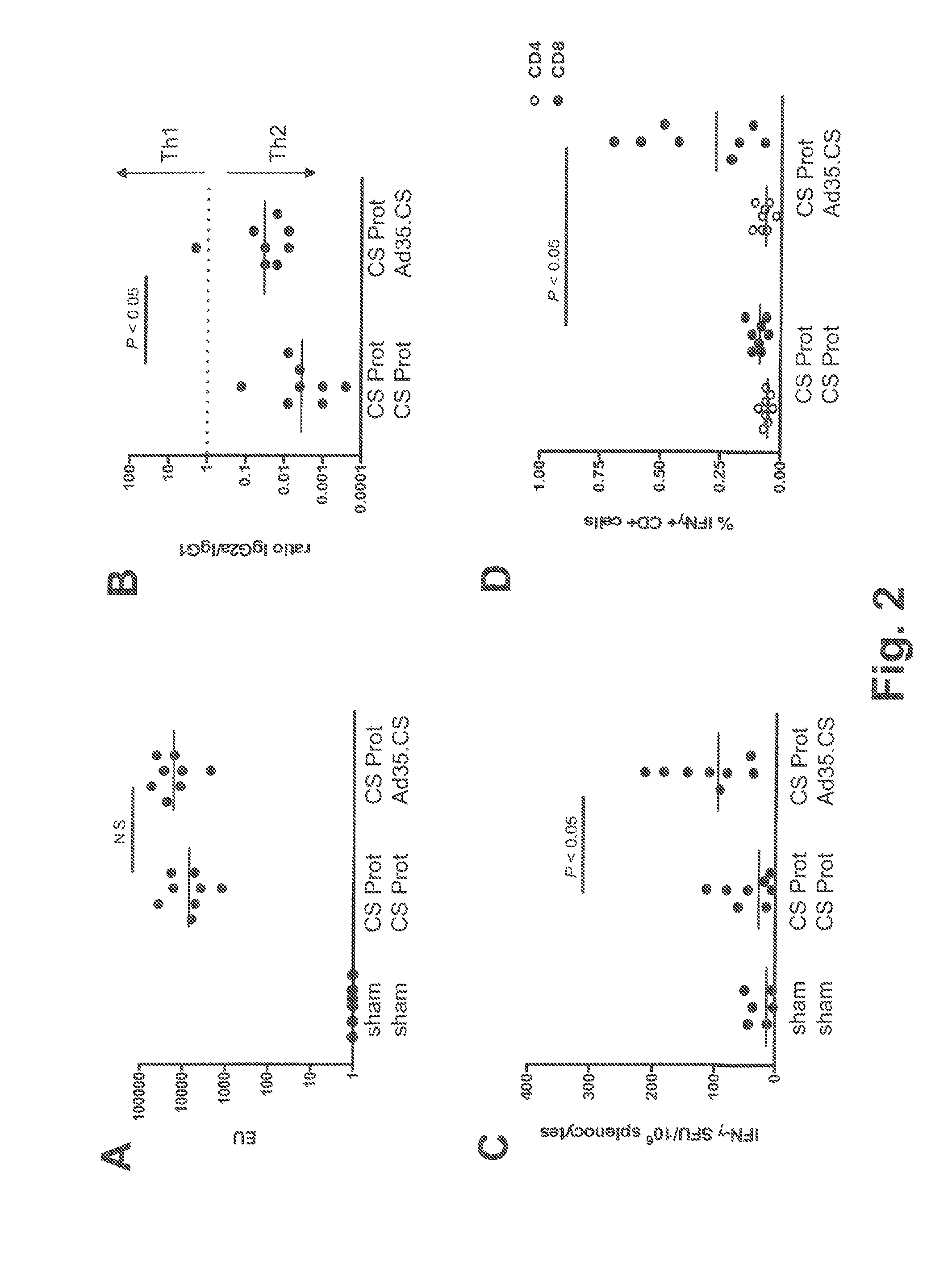
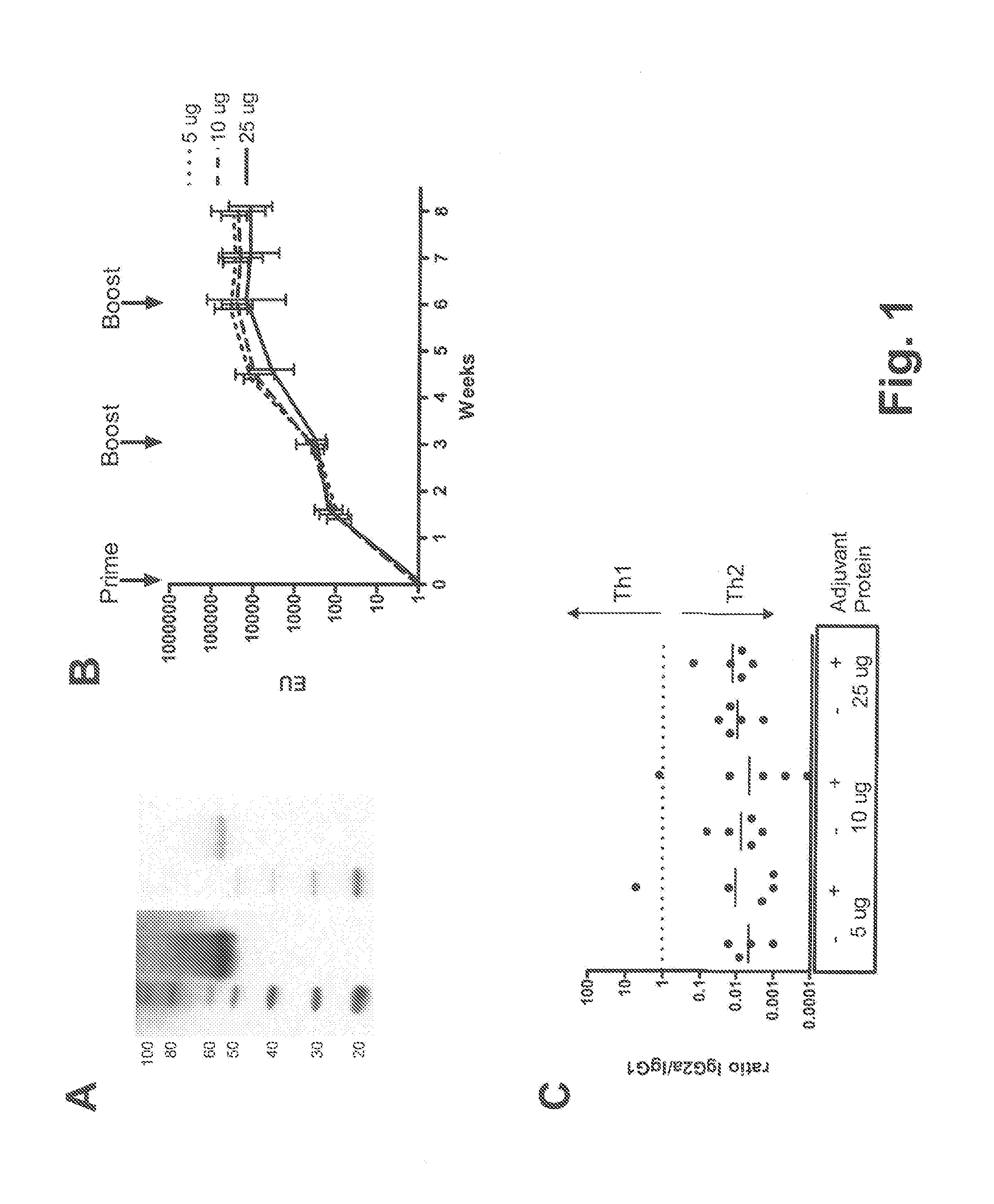
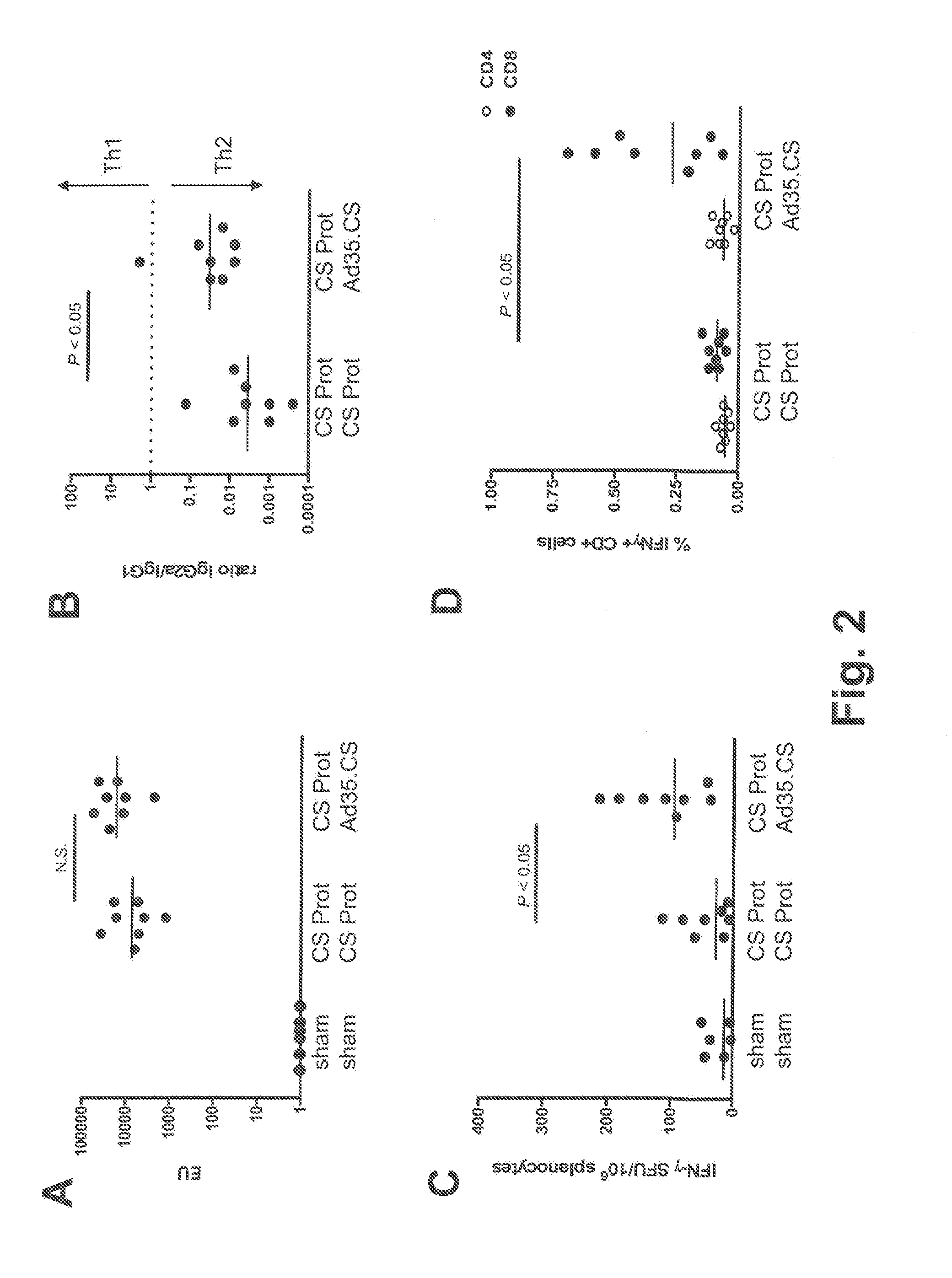
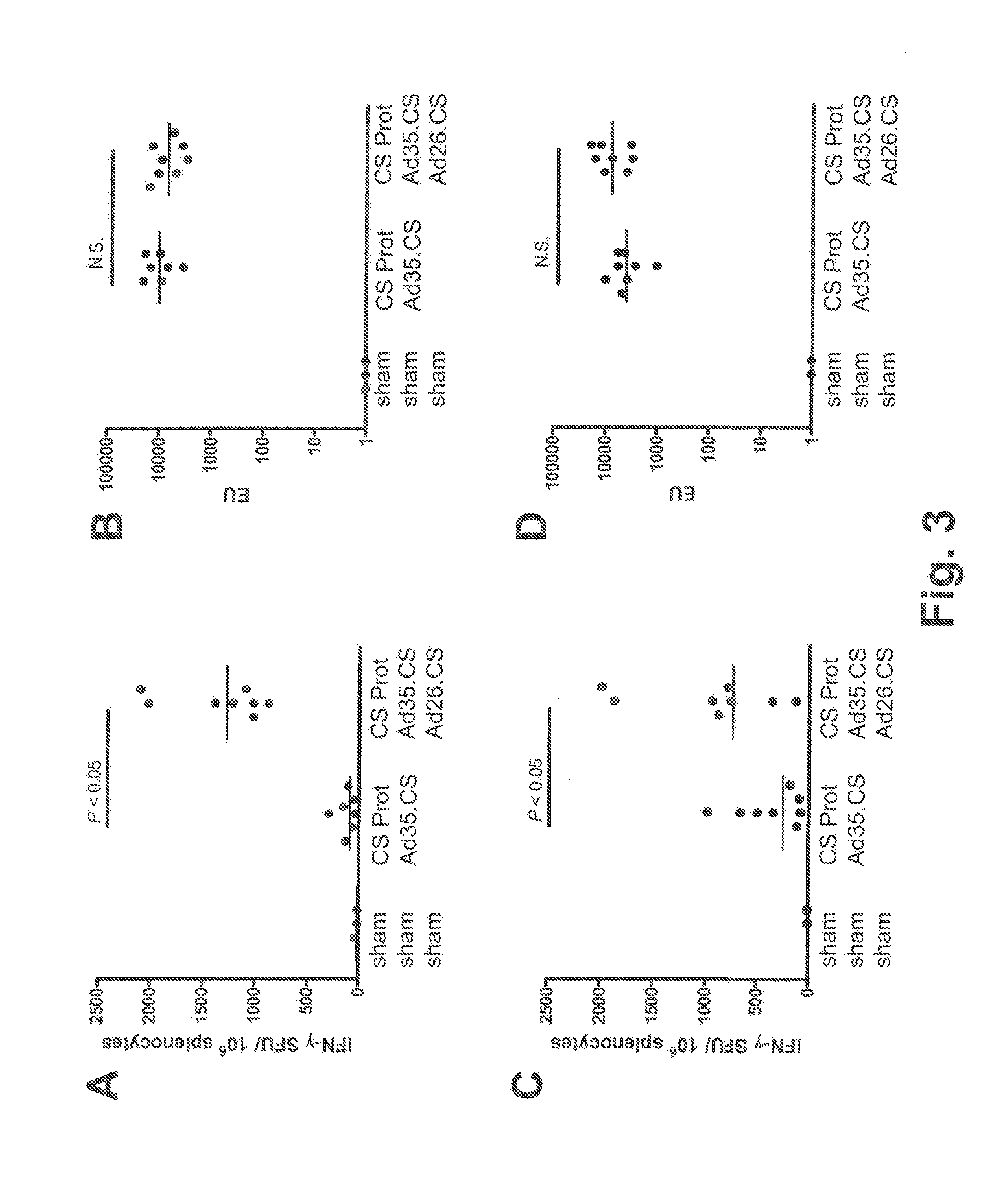
Described are methods for inducing an immune response in a subject against an antigen from a malaria-causing parasite, preferably P. falciparum, the method comprising: (i) administering to a subject a priming composition comprising adjuvanted proteinaceous antigen-comprising circumsporozoite (CS) protein or an immunogenic part thereof from a malaria-causing parasite; (ii) administering to the subject a first boosting composition comprising a recombinant adenovirus vector that comprises nucleic acid encoding CS protein or immunogenic part thereof from a malaria-causing parasite; and (iii) administering to the subject a second boosting composition comprising a recombinant adenovirus vector that comprises nucleic acid encoding CS protein or an immunogenic part thereof from a malaria-causing parasite, wherein either the first boosting composition comprises a recombinant adenovirus vector of serotype 35 (Ad35) and the second boosting composition comprises a recombinant adenovirus of Ad26, or wherein the first boosting composition comprises a recombinant adenovirus vector of Ad26 and the second boosting composition comprises a recombinant adenovirus of Ad35.
Owner:JANSSEN VACCINES & PREVENTION BV
Heterologous prime boost vaccination regimen against malaria
ActiveUS20130216580A1High protection levelHigh levelProtozoa antigen ingredientsDsDNA virusesRegimenSerotype
Described are methods for inducing an immune response in a subject against an antigen from a malaria-causing parasite, preferably P. falciparum, the method comprising: (i) administering to a subject a priming composition comprising adjuvanted proteinaceous antigen-comprising circumsporozoite (CS) protein or an immunogenic part thereof from a malaria-causing parasite; (ii) administering to the subject a first boosting composition comprising a recombinant adenovirus vector that comprises nucleic acid encoding CS protein or immunogenic part thereof from a malaria-causing parasite; and (iii) administering to the subject a second boosting composition comprising a recombinant adenovirus vector that comprises nucleic acid encoding CS protein or an immunogenic part thereof from a malaria-causing parasite, wherein either the first boosting composition comprises a recombinant adenovirus vector of serotype 35 (Ad35) and the second boosting composition comprises a recombinant adenovirus of Ad26, or wherein the first boosting composition comprises a recombinant adenovirus vector of Ad26 and the second boosting composition comprises a recombinant adenovirus of Ad35.
Owner:JANSSEN VACCINES & PREVENTION BV
Communication system, communication device and flow control based on status information of data buffer usage
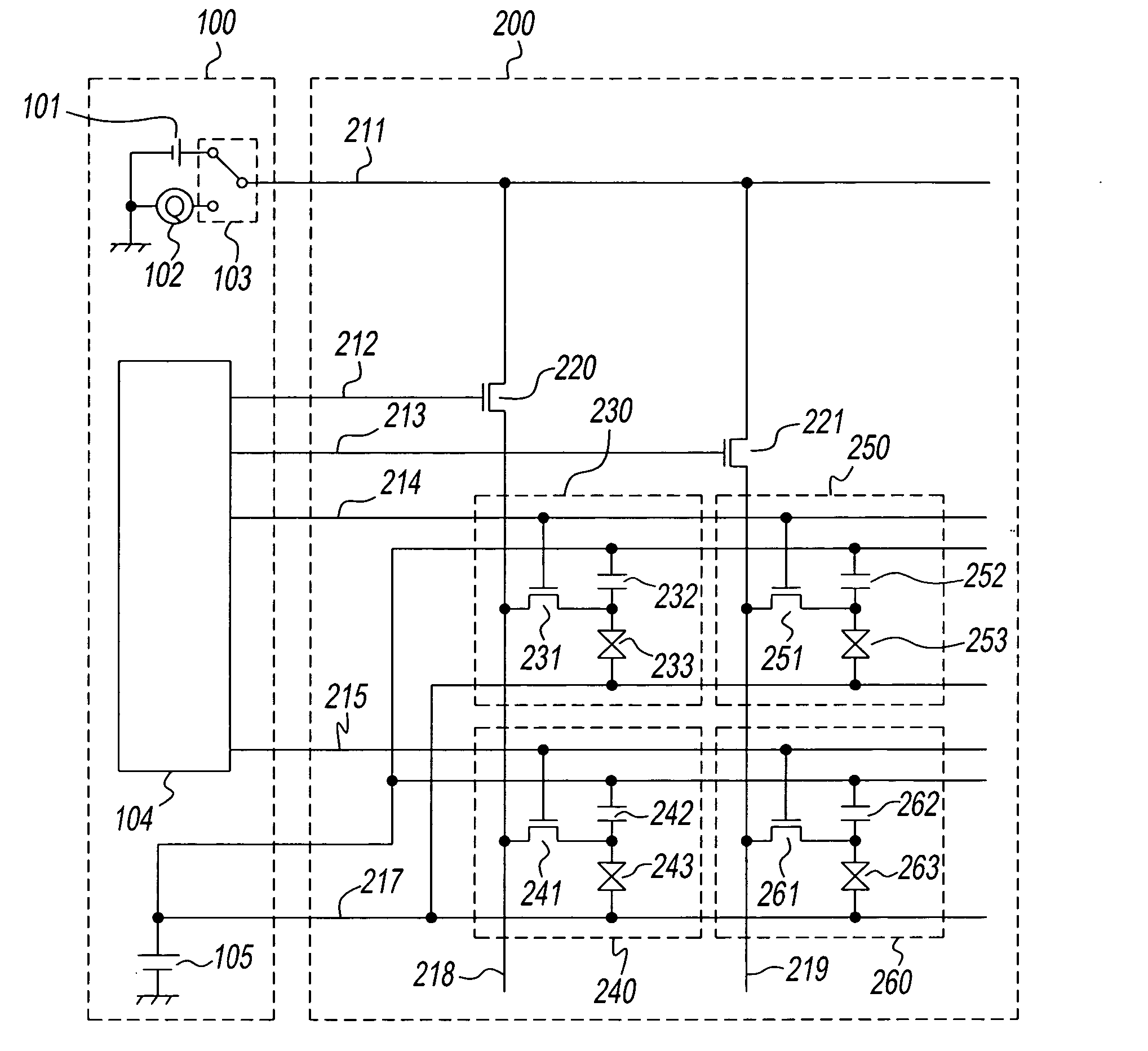
InactiveUS7765335B2Poor responseHigh data efficiencyTransmissionInput/output processes for data processingCommunications systemCommunication device
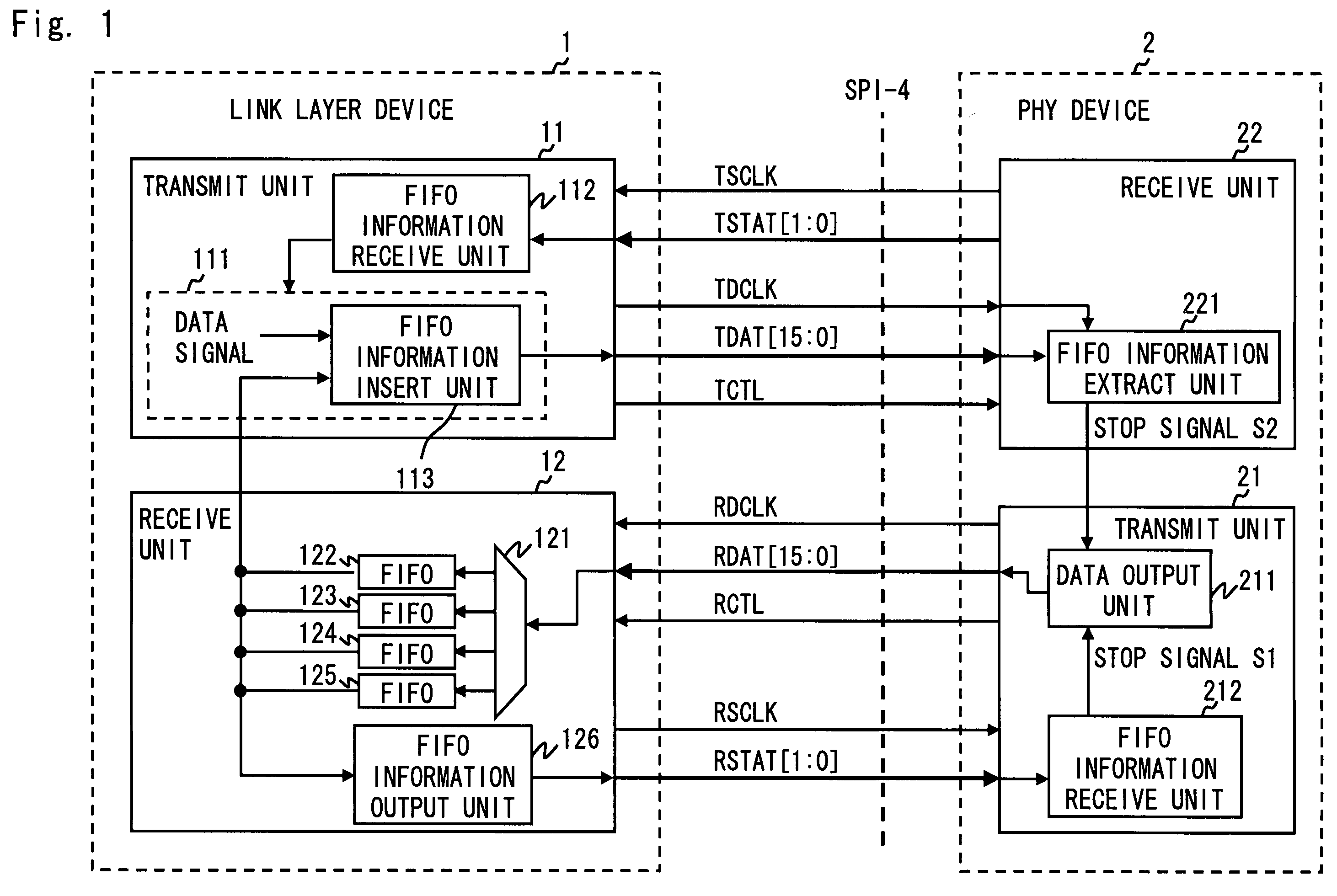
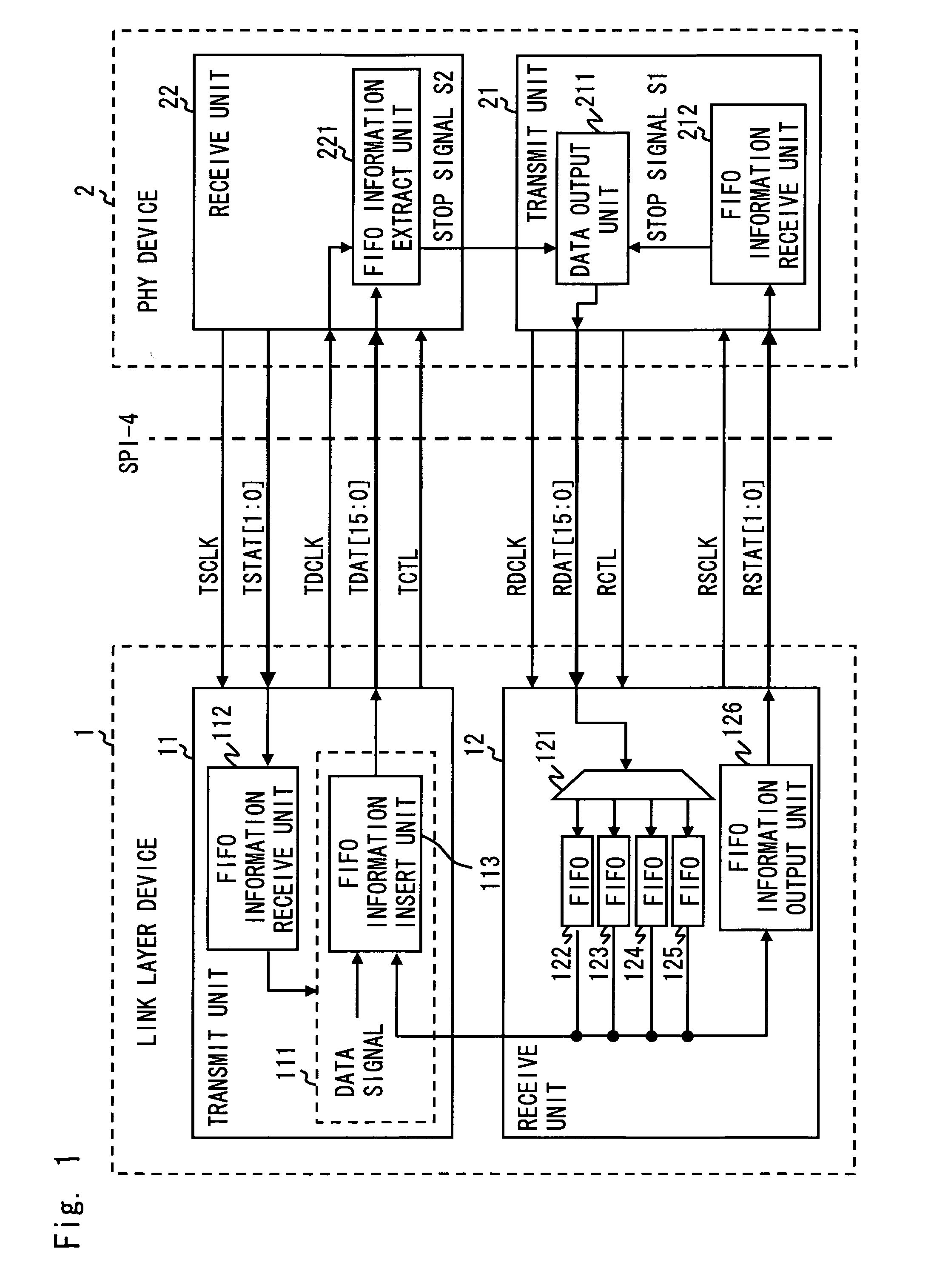
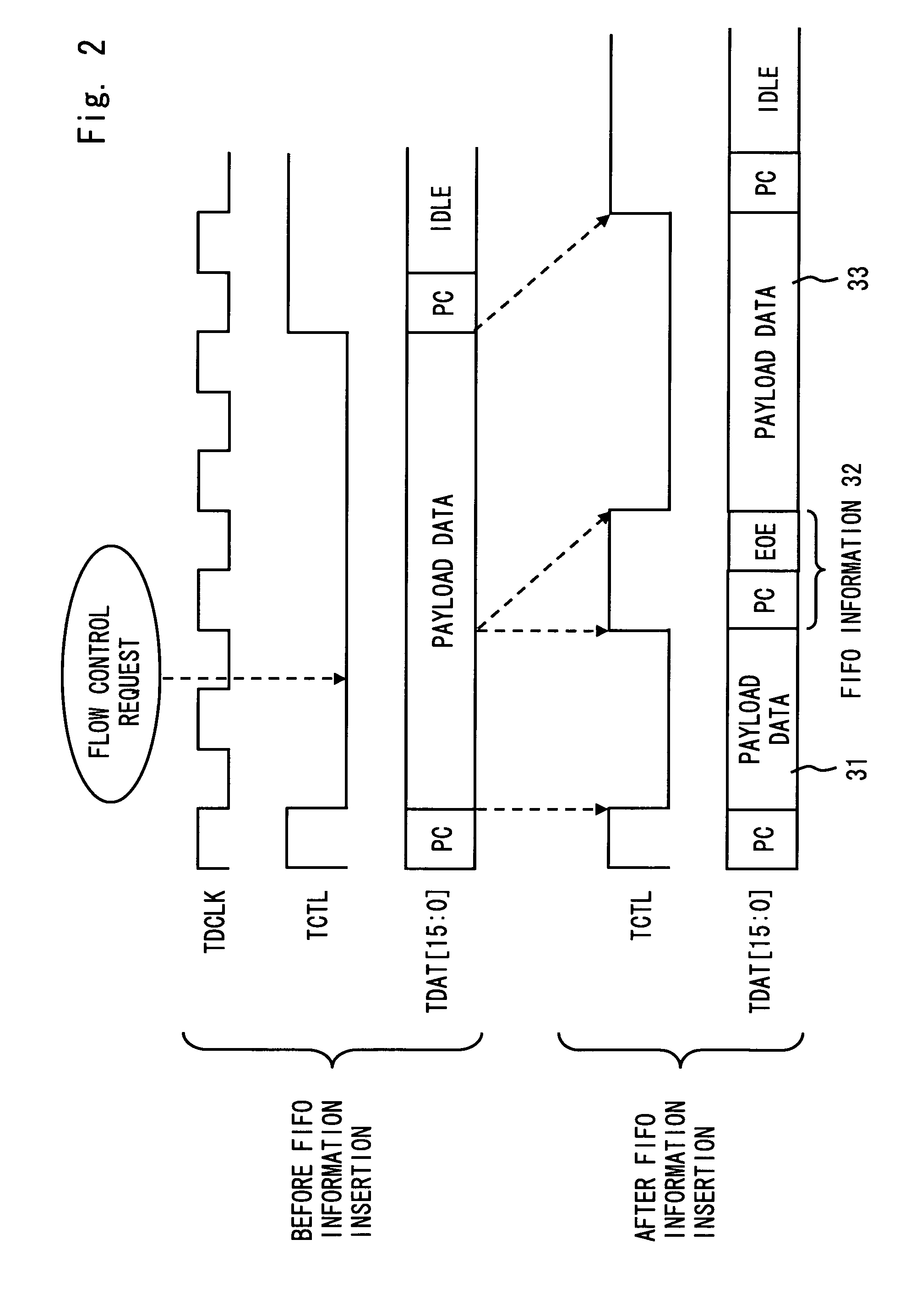
A communication system complying with SPI-4 Phase 2 standard includes a local device, an opposing device, a first data channel to transfer payload data from the local to the opposing device, a second data channel opposed to the first data channel, and a first status channel to be able to transfer data from the local to the opposing device. The local device periodically outputs buffer status information of a data buffer for storing payload data received over the second data channel to the first status channel. Further, the local device inserts the buffer status information between the payload data according to a priority of the buffer status information in order to output the buffer status information to the first data channel. The opposing device controls to output payload data to the second data channel according to the buffer status information received over the first status channel and the first data channel.
Owner:RENESAS ELECTRONICS CORP
Compositions for chimeric antigen receptor t cell therapy and uses thereof
InactiveUS20200230221A1Expands CAR-TFunction increaseTumor rejection antigen precursorsAntibody mimetics/scaffoldsHydrophilic polymersAntigen receptors
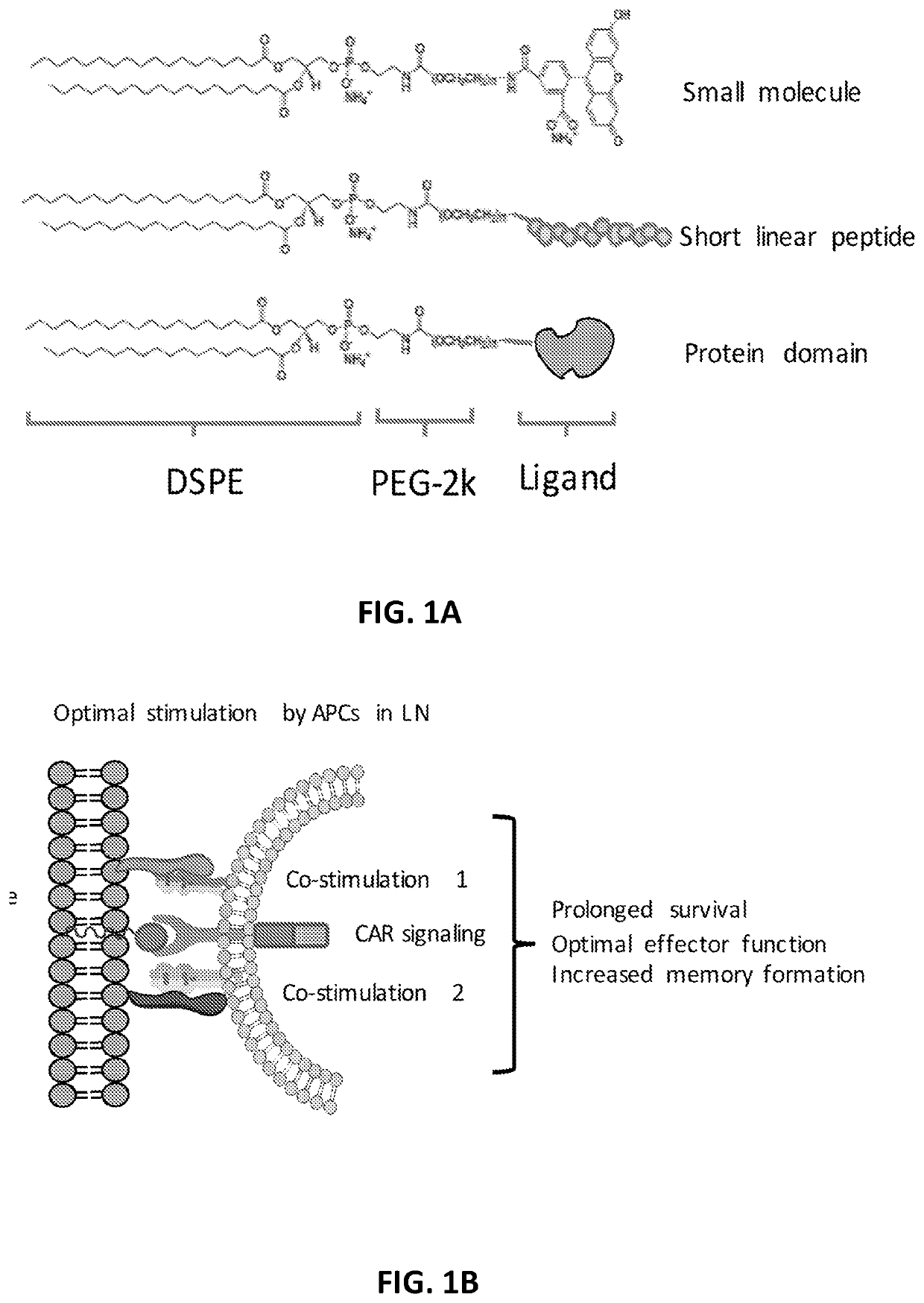
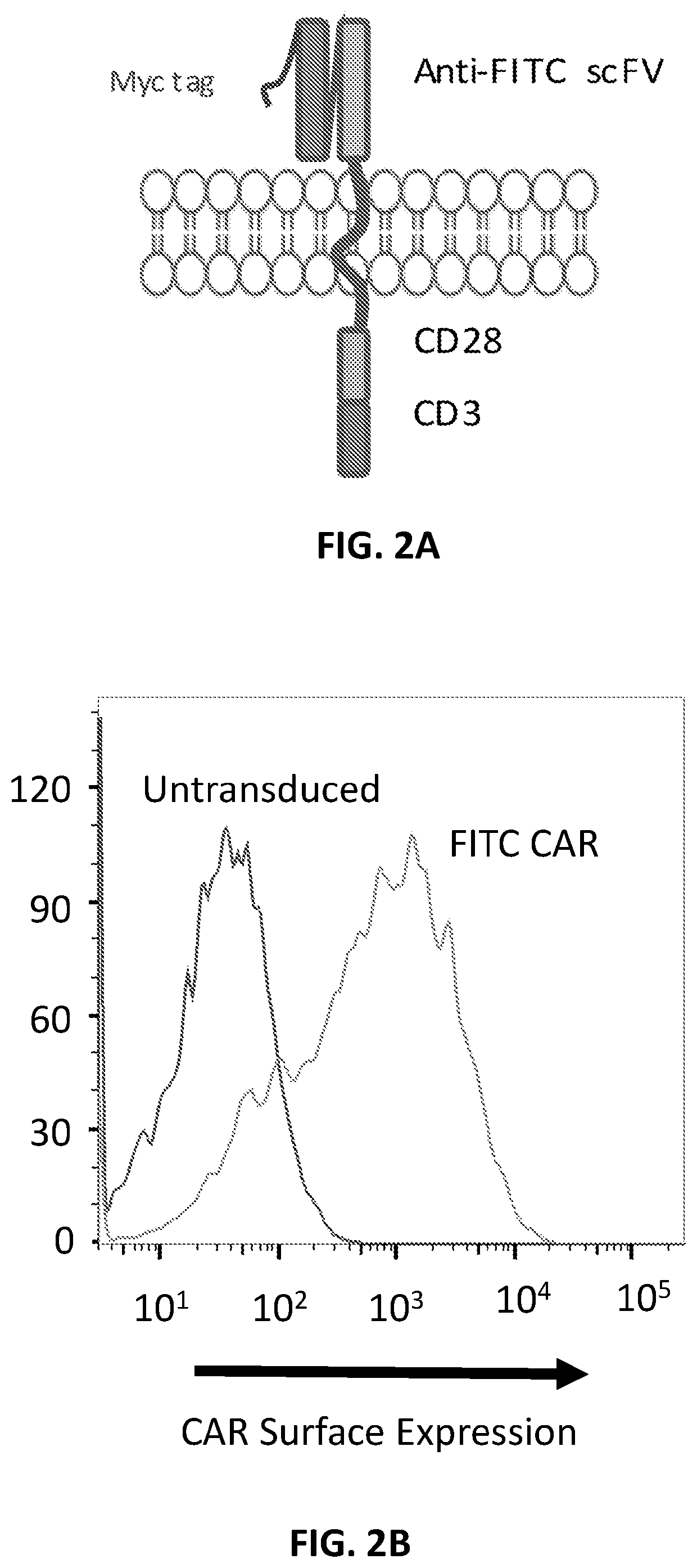
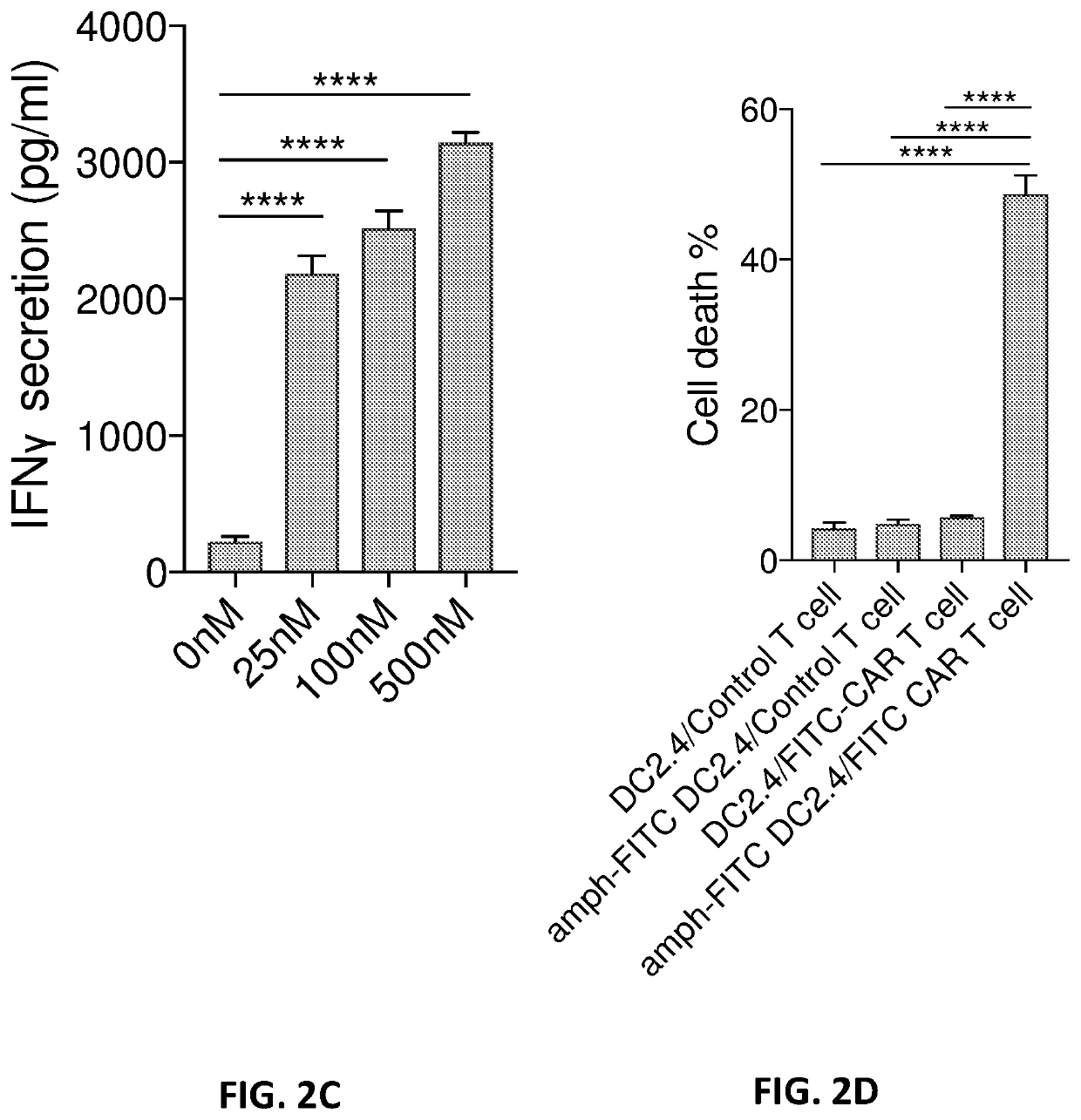
The disclosure describes amphiphilic ligand conjugates comprising a chimeric antigen receptor (CAR) ligand, a lipid (diacyl lipid), a linker (hydrophilic polymers, hydrophilic amino acids, polysaccharides), compositions and methods of using the constructs are claimed, for example, to stimulate proliferation of CAR expressing cells.
Owner:MASSACHUSETTS INST OF TECH
Preparation method and application of palm oil fatty acid calcium
ActiveCN111909026AReduce dosageRapid responseOrganic compound preparationAccessory food factorsFood gradeEmulsion
The invention discloses a preparation method and application of palm oil fatty acid calcium. The method comprises the following steps: (1) mixing palm oil with a food-grade lime emulsion, adding beewax, strongly pulping and dispersing for 2 hours, and dropwisely adding a 90% ethanol solution of sodium ethoxide; (2) heating to a reflux state, keeping the reflux state for 2-4h, and carrying out ultrasonic treatment by using an ultrasonic reactor; and (3) carrying out reduced pressure distillation on the ethanol solution to remove ethanol and to obtain a concentrate, performing filtration to remove most of water, drying, and carrying out vacuum drying on the concentrate at 60 DEG C for 6 hours to obtain the palm oil fatty acid calcium.
Owner:BELIKE CHEM
Melt extruded fibers and methods of making the same
InactiveUS20070154708A1Melt viscosityPoor responseSpinnerette packsSpinning head liquid feederFiberPolymer science
Polymeric fibers along with methods and systems for extruding polymeric fibers are disclosed. The extrusion process preferably involves the delivery of a lubricant separately from a polymer melt stream to each orifice of an extrusion die such that the lubricant preferably encases the polymer melt stream as it passes through the die orifice.
Owner:3M INNOVATIVE PROPERTIES CO
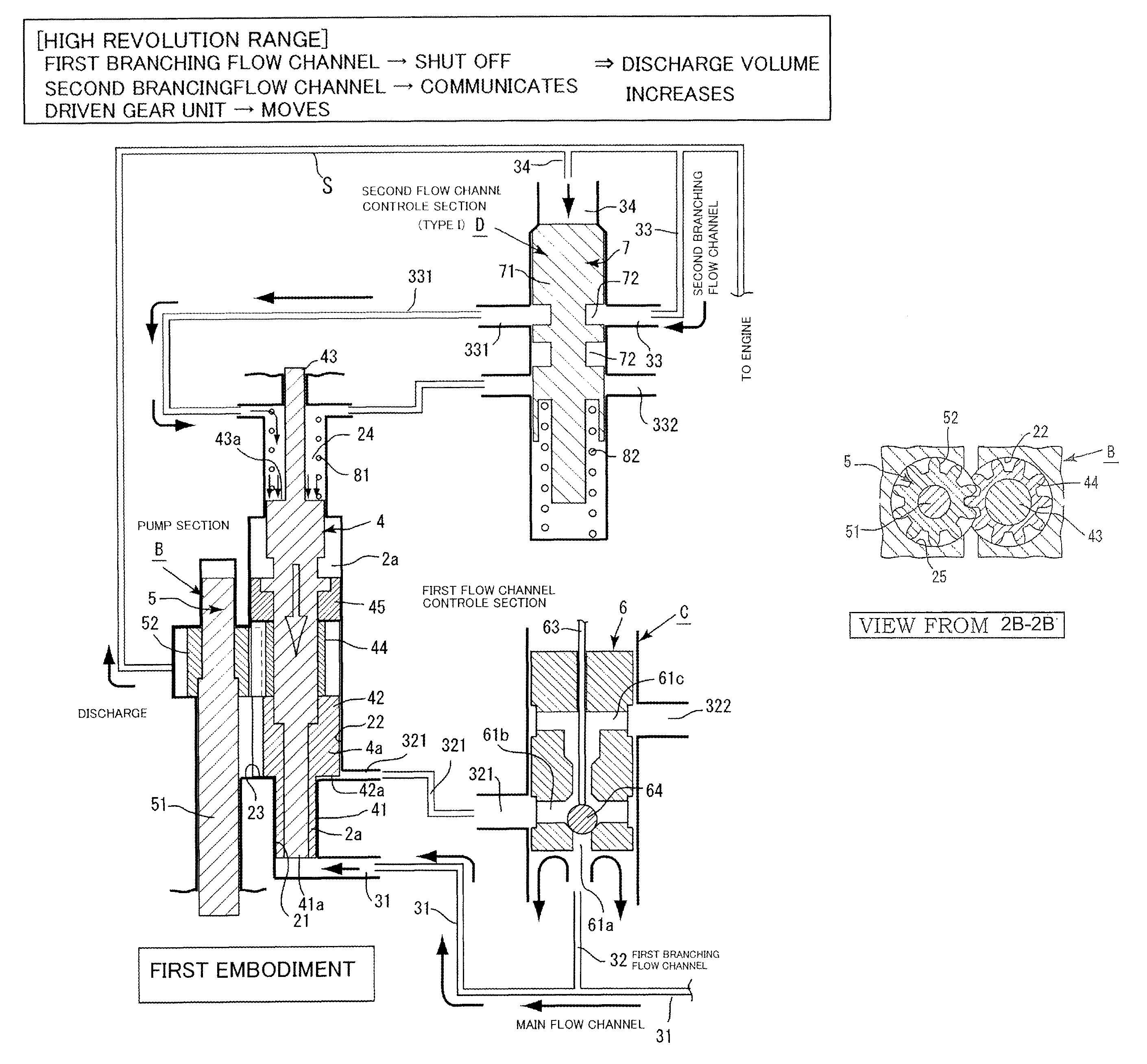
Pump device
InactiveUS8926299B2Poor responseMinimal loadingOscillating piston enginesEngine of intermeshing engagement typeEngineeringMechanical engineering
The invention has: a housing; a pump section formed of a drive gear unit and a driven gear unit; a main flow channel through which oil pressure is applied to the driven gear unit in a discharge volume reduction direction; a first branching flow channel through which oil pressure that assists oil pressure from the main flow channel is applied; a second branching flow channel through which oil pressure is applied to the driven gear unit in a discharge increase direction; a first flow channel control section; a second flow channel control section; and a spring that elastically urges the driven gear unit in a discharge increase direction. The first flow channel control section and the second flow channel control section can perform switching control in accordance with each increase or decrease of engine revolutions and in pressure.
Owner:YAMADA MANUFACTURING CO LTD
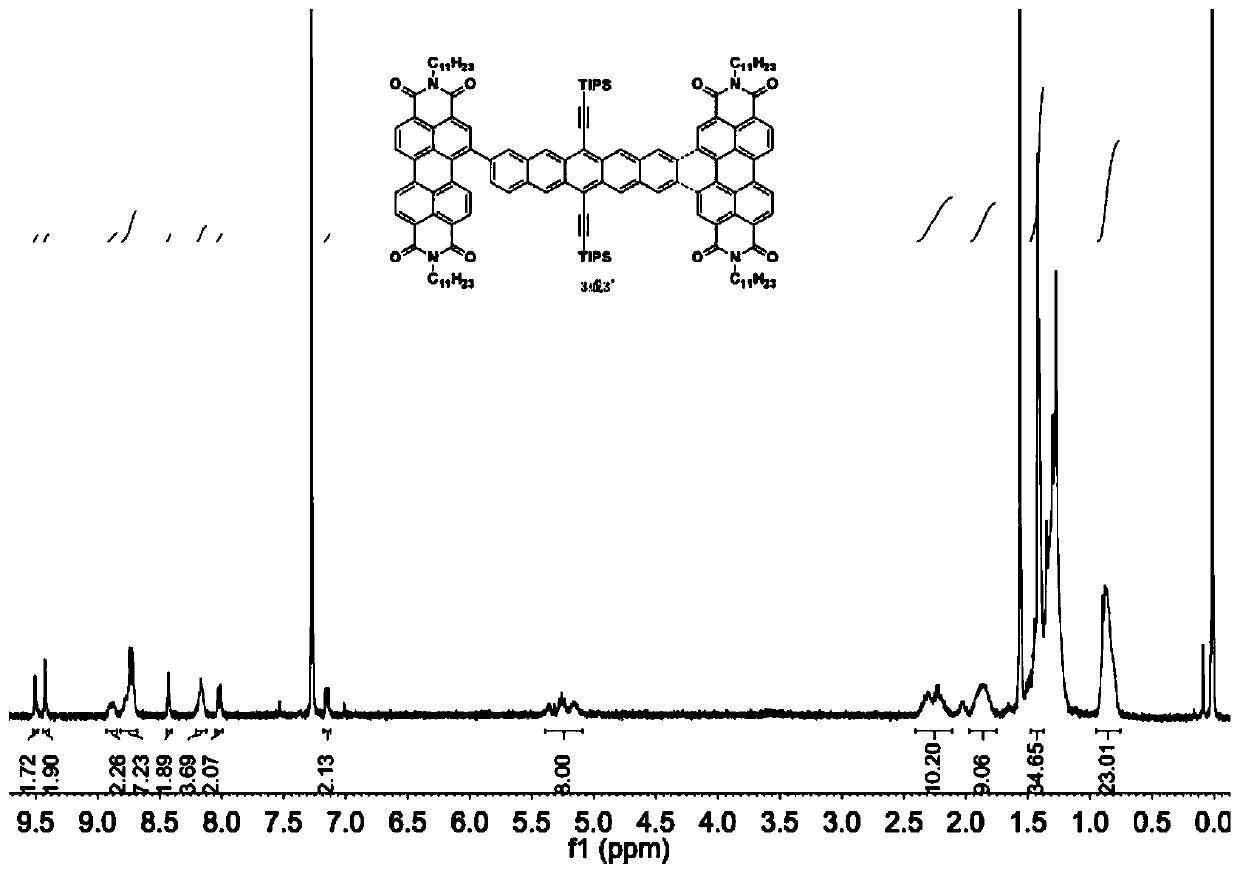
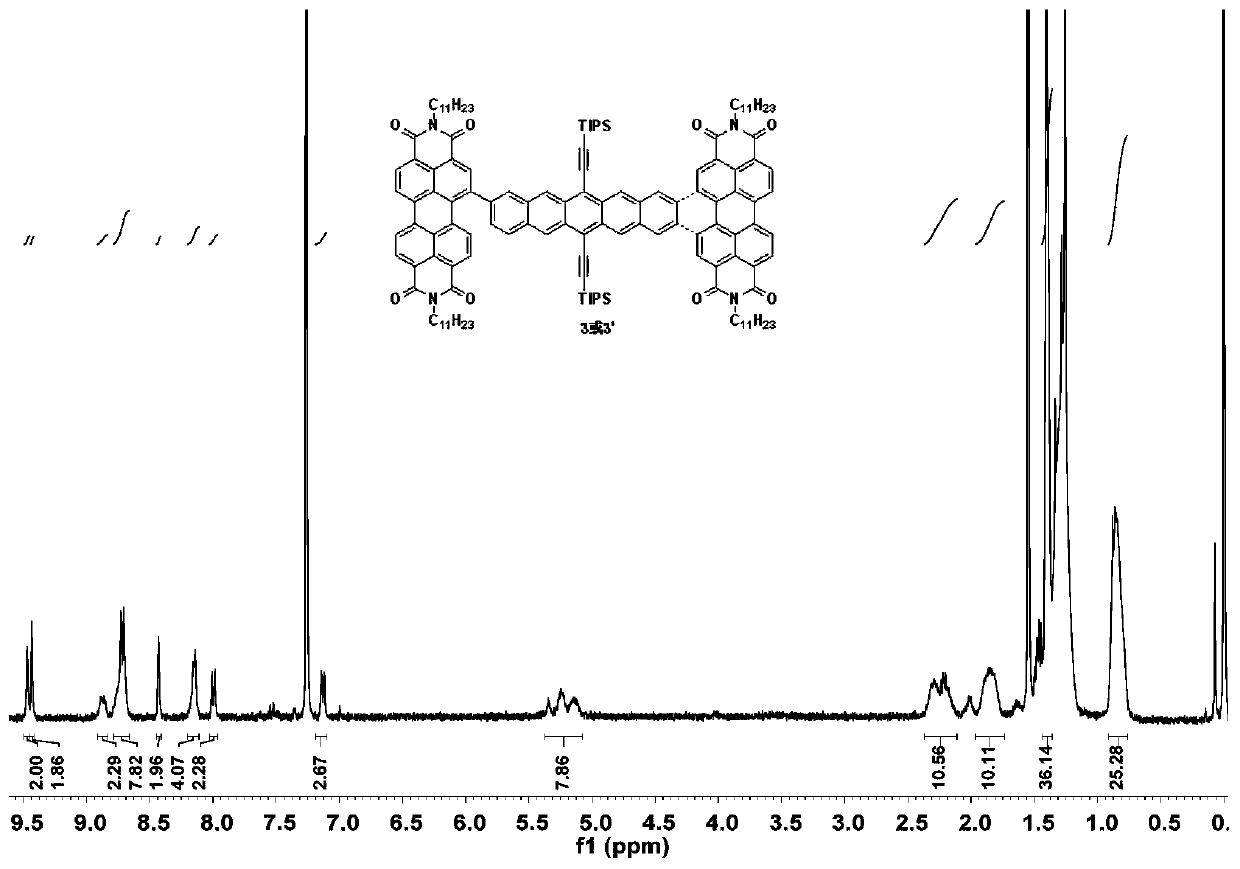
Synthetic method of A-D-A organic photoelectric micro-molecule based on perylene diimide and pentacene
ActiveCN107793444AImprove efficiencyGood singlet fission propertiesSilicon organic compoundsSolid-state devicesSolventBromine
The invention relates to a synthetic method of an A-D-A organic photoelectric micro-molecule based on perylene diimide and pentacene. The synthetic method includes the steps: dissolving 6, 13-bi (triisopropyl silicon acetenyl) pentacene-2, 9-bi-pinacol ester or 6, 13-bi (triisopropyl silicon acetenyl) pentacene-2, 10-bi-pinacol ester and 1-bromine perylene diimide in solvents under inert atmosphere; performing coupled reaction under the action of catalysts to obtain 2, 10-biperylene diimide-6, 13-bi (triisopropyl silicon acetenyl) pentacene or 2, 9-biperylene diimide-6, 13-bi (triisopropyl silicon acetenyl) pentacene; performing LED (light-emitting diode) lamp illumination and photocycloaddition reaction under the action of iodine elementary substances to prepare the micro-molecule. According to the method, electron acceptor materials with excellent performances and singlet-state fission model molecules are firstly assembled into one molecule, and the molecule is applied to fields suchas organic solar cells, field effect transistors, photoelectric detectors and organic light emitting diodes.
Owner:SHANGHAI NORMAL UNIVERSITY
Exposure apparatus
InactiveUS7325404B2Good optical performanceImprove throughput performanceDomestic cooling apparatusSemiconductor/solid-state device manufacturingMechanical engineeringEngineering
An exposure apparatus includes an optical system for guiding light to an object, a holding member for holding the object, a first refrigerator located near a holding side of the holding member without contacting the holding side, and a second refrigerator located near a backside of the holding member without contacting the backside.
Owner:CANON KK
Composition and Method for Improving Sleep Duration and Quality
ActiveUS20180021411A1Large dropout ratePoor responsePeptide/protein ingredientsHeavy metal compound active ingredientsSide effectPhysiology
An intervention for sleep disorders comprises: collagen, a gelatin peptide, or the amino acid glycine; L-theanine; lactucopicrin, deoxylactucopicrin, or another lactucopicrin derivative; hyaluronic acid; epigallocatechin gallate; and quinic acid. The intervention helps regulate pro-inflammatory biomarkers that are often associated with insomnia development and progression. These biomarkers include cytokines and enzymes associated with tryptophan degradation. Inhibition of these enzymes and cytokines improves tryptophan availability and sleep quality, and allows individuals to sleep better with fewer side effects. The composition promotes high-quality, deep sleep.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Production process of evodiamine and method for recycling solvent in production
InactiveCN112390798AReduce usageReduce security risksOrganic compound preparationCarboxylic acid esters preparationChemistryOrganic synthesis
The invention relates to the field of organic synthesis, in particular to a production process of evodiamine and a method for recycling a solvent in production. According to the method, tryptamine isadopted as a raw material, evodiamine is obtained through formylation, cyclization and condensation ring closing, phosgene and ethyl chloroformate are prevented from being used in the whole process, the safety risk in the production process is reduced, and the reaction steps are greatly simplified. Meanwhile, a solvent application process in production is realized, so that the production cost is greatly reduced, and the method has an industrial popularization value.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Prognostic and treatment response predictive method
PendingUS20210139999A1Easy to sign forImprove predictive performanceMicrobiological testing/measurementBiostatisticsCCL2IL12A
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of at least 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL A in a sample obtained from the tumour of the patient; b) measuring the gene expression of at least 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained from the tumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including with immune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Micro-fluidic chip device for biogenic amine detection and application
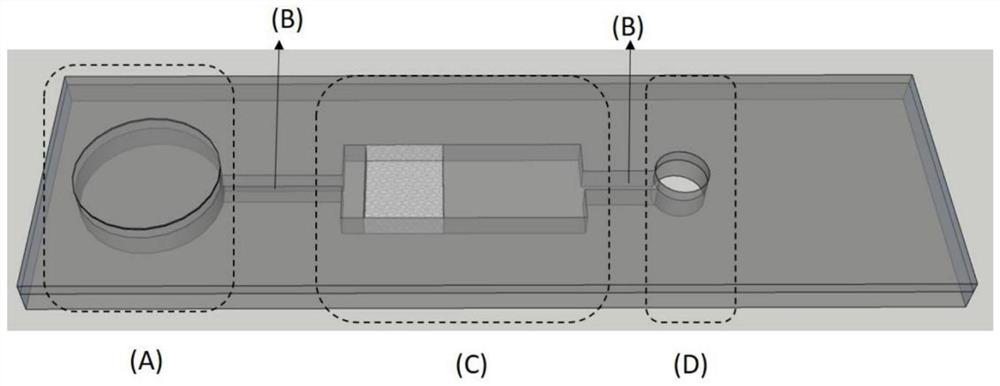
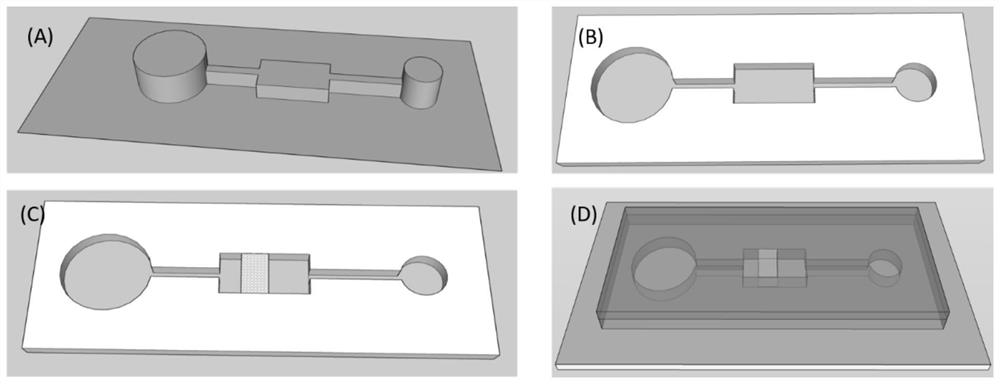
PendingCN114088697APoor responseImprove detection accuracyMaterial analysis by observing effect on chemical indicatorLaboratory glasswaresFood industryCellulose acetate
The invention discloses a micro-fluidic chip device for biogenic amine detection and application. The micro-fluidic chip device comprises a sample inlet, a connecting channel, a detection module and an air pressure driving area; the sample inlet, the detection module and the air pressure driving area are sequentially connected through the connecting channel; the sample inlet is connected with the atmosphere; the detection module comprises a cellulose acetate-based fluorescent substance modified three-dimensional porous polydimethylsiloxane (PDMS) structure, and the air pressure driving area provides negative pressure in a pressing mode to promote to-be-detected gas or liquid outside the chip to enter the chip. The food freshness can be judged by detecting the response conditions of biogenic amines in different foods. The prepared micro-fluidic device has quick and reversible response to biogenic amine, so that the micro-fluidic device can be easily used as a quick detection device and can be practically applied to the food industry.
Owner:NANTONG UNIVERSITY
A kind of preparation method and application of palm oil fatty acid calcium
ActiveCN111909026BReduce dosageRapid responseOrganic compound preparationAccessory food factorsFood gradeEmulsion
The invention discloses a preparation method and application of palm oil fatty acid calcium. The method comprises the following steps: (1) mixing palm oil with a food-grade lime emulsion, adding beewax, strongly pulping and dispersing for 2 hours, and dropwisely adding a 90% ethanol solution of sodium ethoxide; (2) heating to a reflux state, keeping the reflux state for 2-4h, and carrying out ultrasonic treatment by using an ultrasonic reactor; and (3) carrying out reduced pressure distillation on the ethanol solution to remove ethanol and to obtain a concentrate, performing filtration to remove most of water, drying, and carrying out vacuum drying on the concentrate at 60 DEG C for 6 hours to obtain the palm oil fatty acid calcium.
Owner:BELIKE CHEM
Compositions and methods for sars-2 vaccine with virus replicative particles and recombinant glycoproteins
PendingUS20220193225A1Poor iga responsePoor responseSsRNA viruses negative-senseSsRNA viruses positive-senseAdenovirus vaccineMucosal iga
A novel and improved vaccine for prevention of disease caused by the Severe Acute Respiratory Syndrome-2 (SARS-2), / COVID-19 virus. Current mRNA and Adenovirus vaccine technologies for SARS-2 provide high levels of serum Immunoglobin G (IgG), antibodies against the original Wuhan strain, but there are now hundreds of mutant strains which can evade both vaccine and convalescent antibodies. These vaccines also do not provide strong mucosal IgA class antibodies which provide wider protection against mutant strains of Flu A and other respiratory viruses. The ability of these technologies to provide high levels of protection is in question, as serum neutralizing antibodies may decline to undetectable levels after six months. The appearance of mutant strains such as the Beta, Gamma, Delta, and Epsilon strains, containing altered amino acid sequences capable of evading vaccine-induced antibodies, calls for new vaccine technologies that can be quickly altered to meet this threat. The following describes a combination approach to prevention of infection by SARS-2 / COVID-19. This combination consists of a priming injection of Recombinant Replicative Particles (VRP) derived from the Alphavirus Venezuelan Equine Encephalitis (VEE) strain 3000 / 3526, with insertion of a Delta / B.1.617.2 SARS-2 / COVID-19 spike 1 glycoprotein (gp)-Receptor-Binding Domain (RBD) gene. The insertion of Internal Ribosome Entry Sites (IRES), elements between the 26S promoter and the SARS-2 / COVID RBD gene allows for more efficient translation of the SARS-2 / COVID gene products. The VEE3000 / 3256 VRP are produced from plasmids, so while they are infectious for one replicative cycle in vivo, progeny VRP are replication incompetent. The priming is followed by one or more intranasal administrations of a suspension of recombinant SARS-2 / COVID-19 envelope spike 1 glycoproteins (gp), from selected mutant strains, combined with the pulmonary surfactant adjuvant, SF-10. The goal of the invention is to safely provide multiple immune layers of protection in both the upper and lower respiratory tracts, with induction of both mucosal IgA and serum IgG antibodies, as well as effector Cytotoxic T Lymphocyte (CTL), cells recognizing conserved regions of the SARS-2 / COVID-19 virus genome. Secondary goals are to reduce the risk of antibody-dependent enhancement (ADE), of infection, a major concern with other SARS-2 / COVID-19 vaccine designs, and to provide capacity to protect against mutant emergent strains of SARS-2 / COVID-19 with annual intranasal boosters of new spike glycoproteins.
Owner:CORONAVAX LLC
Prognostic Methods in Colorectal Cancer
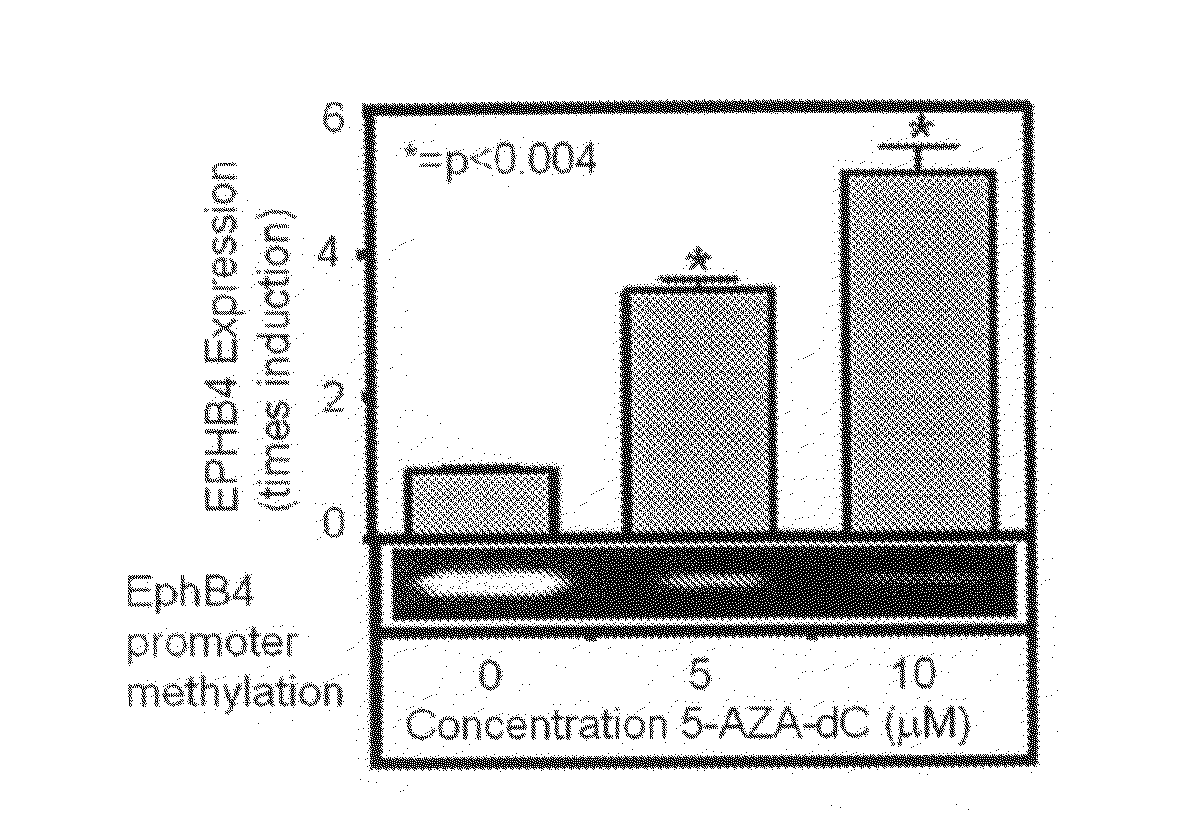
InactiveUS20100035762A1Good pronostic markerLow levelElectrolysis componentsVolume/mass flow measurementProper treatmentWilms' tumor
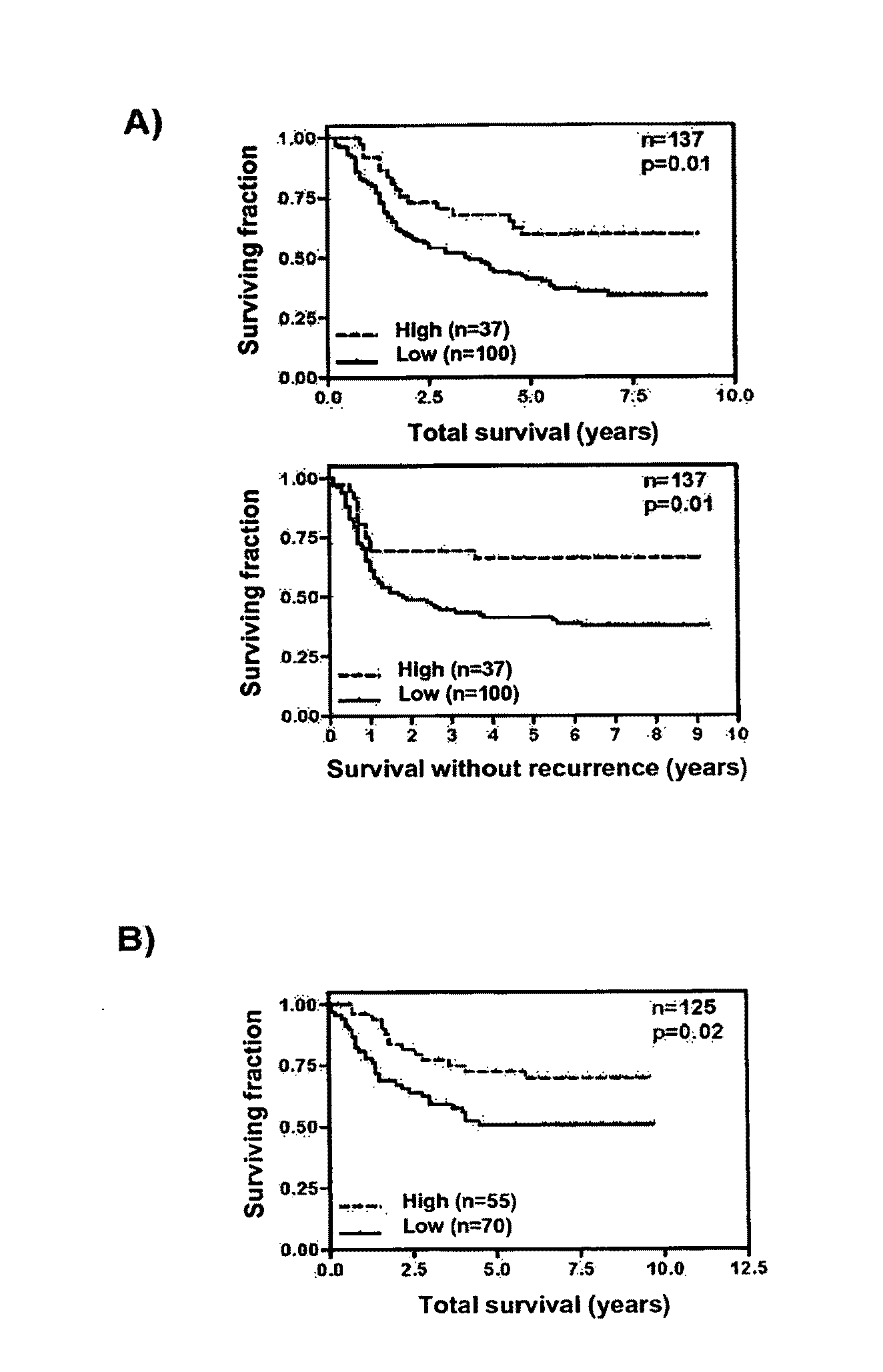
Prognostic method in colorectal cancer based on the determination of the expression levels of the EphB4 gene in tumours of patients afflicted by this disease. The levels can be used as a marker for the probability of the recurrence of the cancer in the patient and for the prognostics of the sensitivity that the tumours present to treatment with 5-fluorouracil, allowing to establish the more adequate therapeutic strategy for every patient.
Owner:ORYZON GENOMICS SA
Manganese superoxide dismutase Val16Ala polymorphism predicts resistance to chemotherapeutic drug cancer therapy
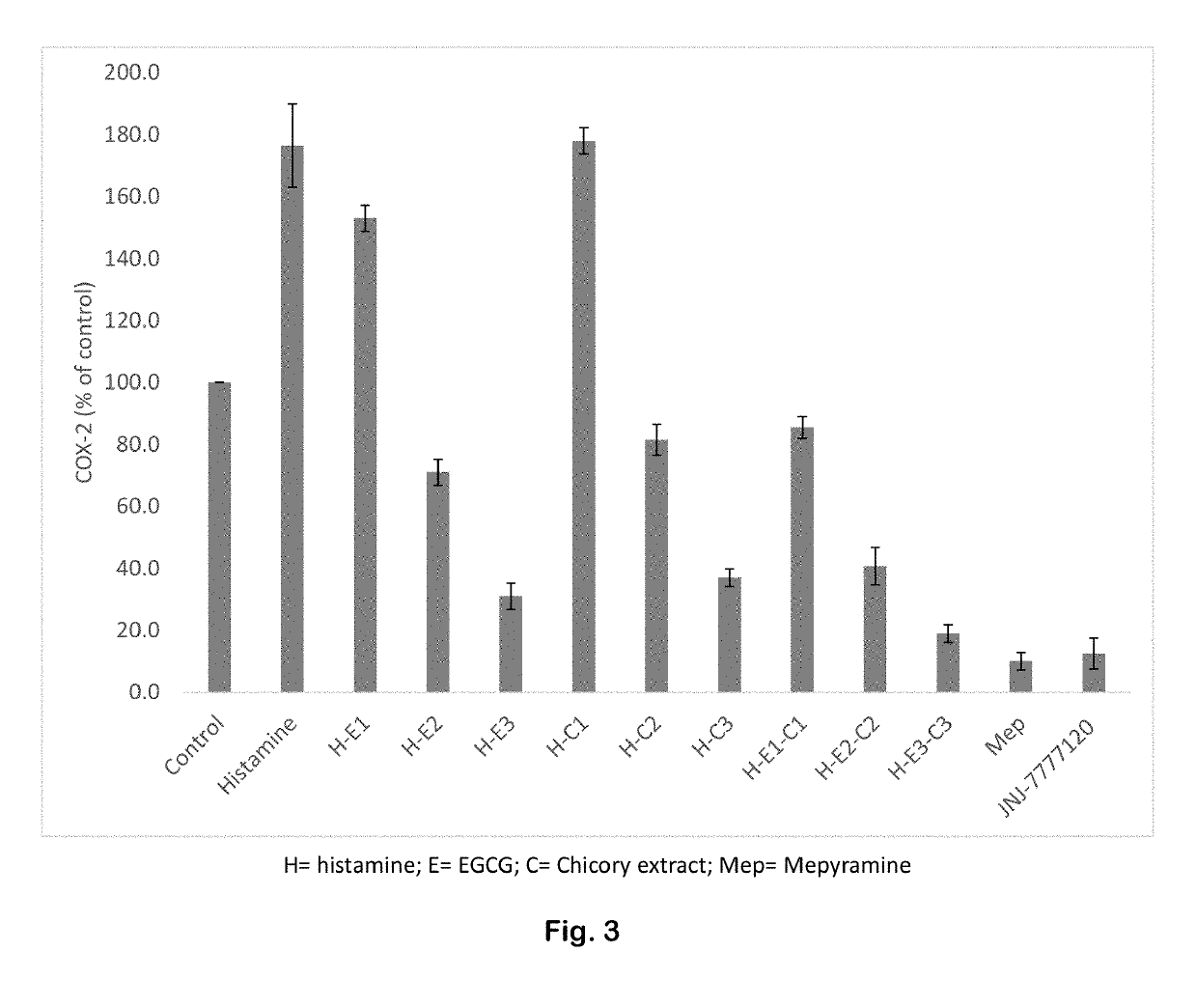
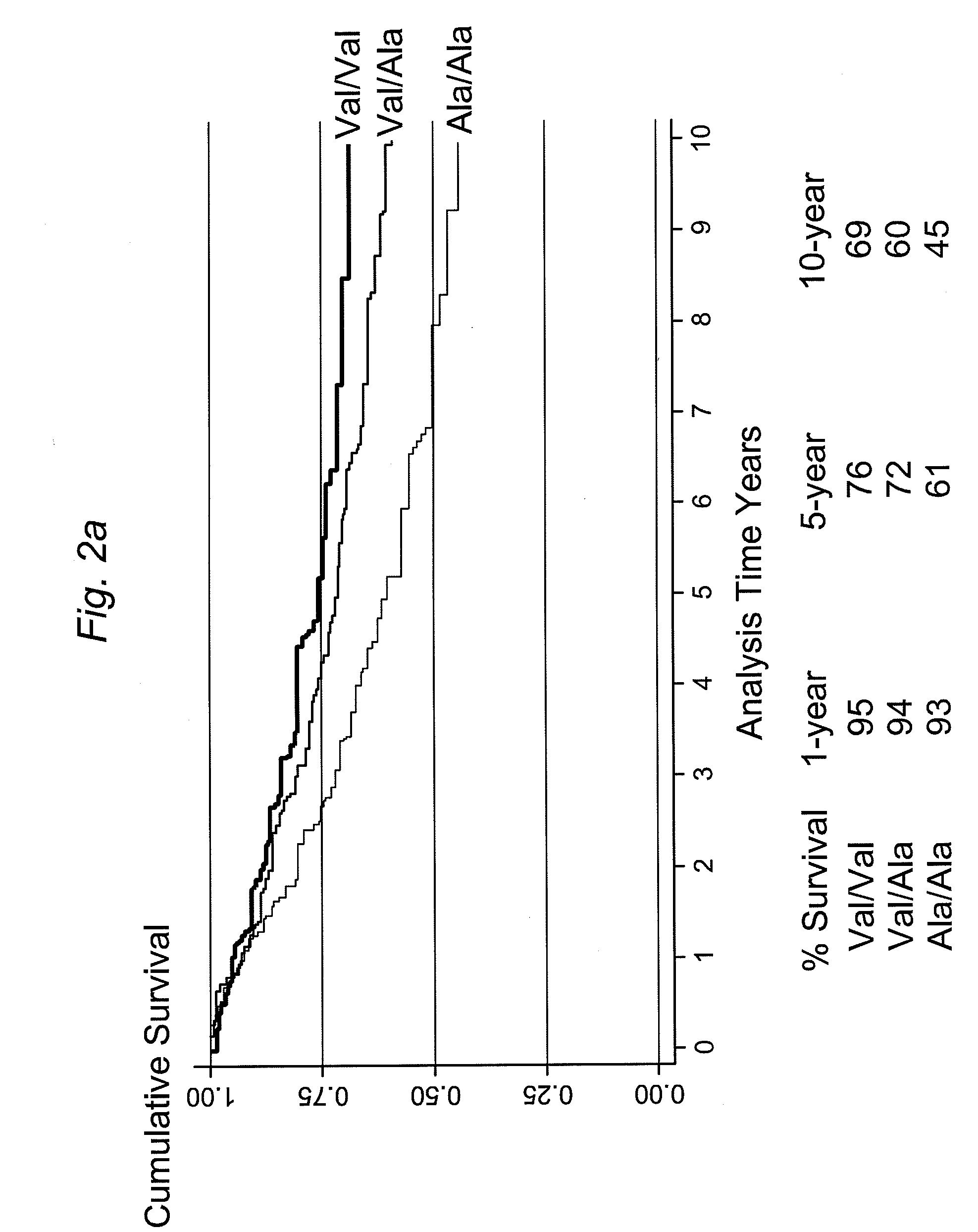
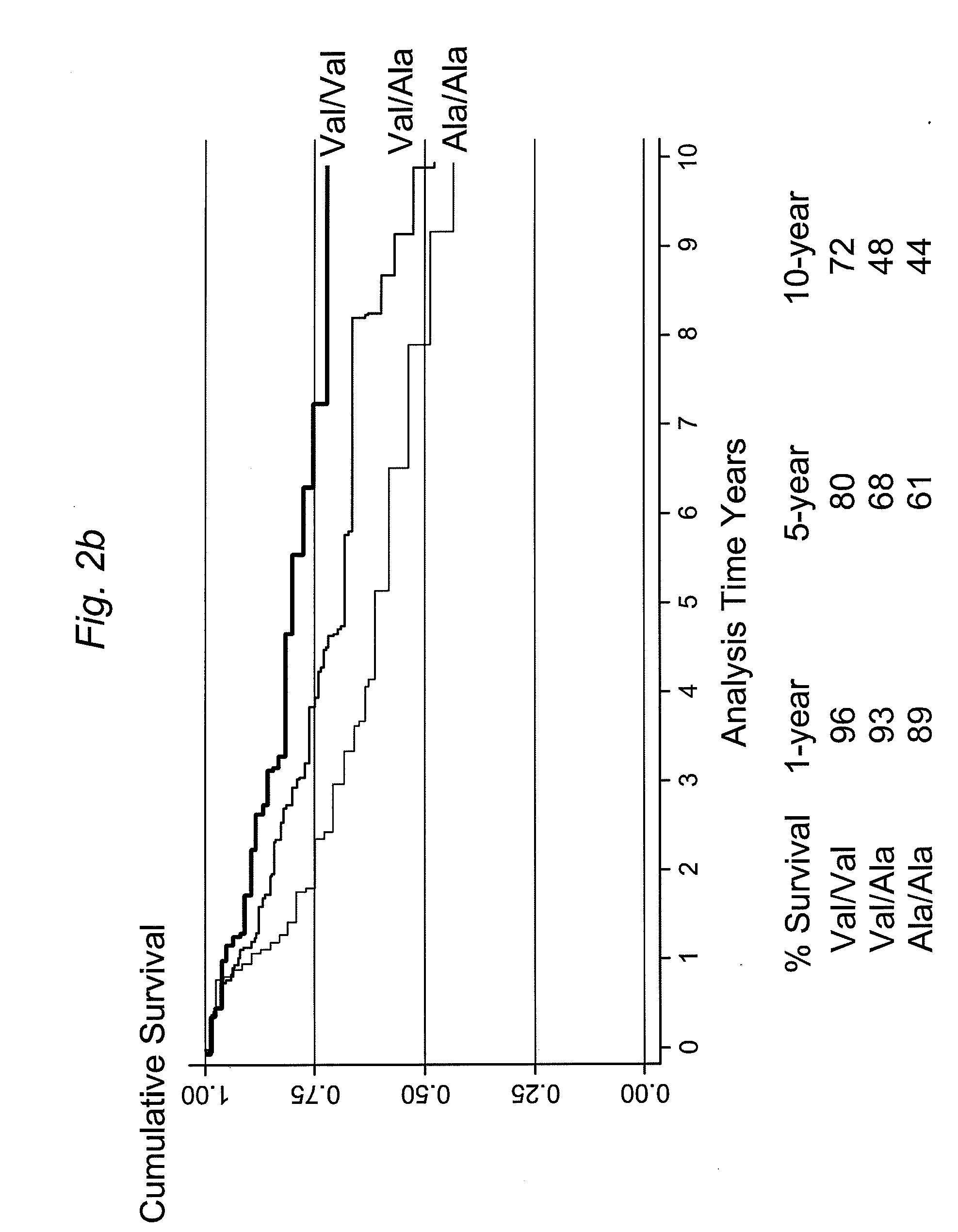
InactiveUS7985561B2Poor responsePoor outcomeMicrobiological testing/measurementBiological testingGenotypeBiomarker (petroleum)
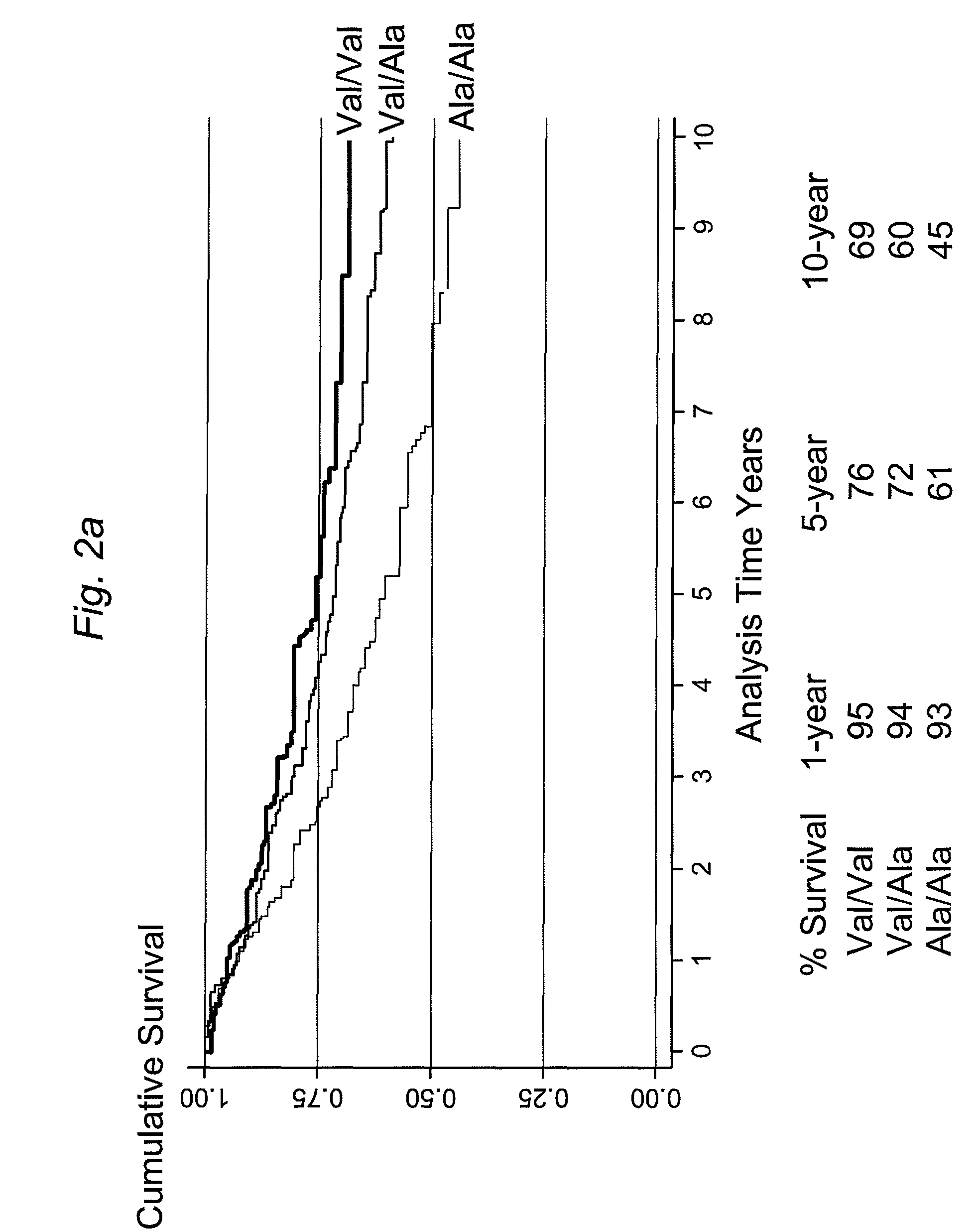
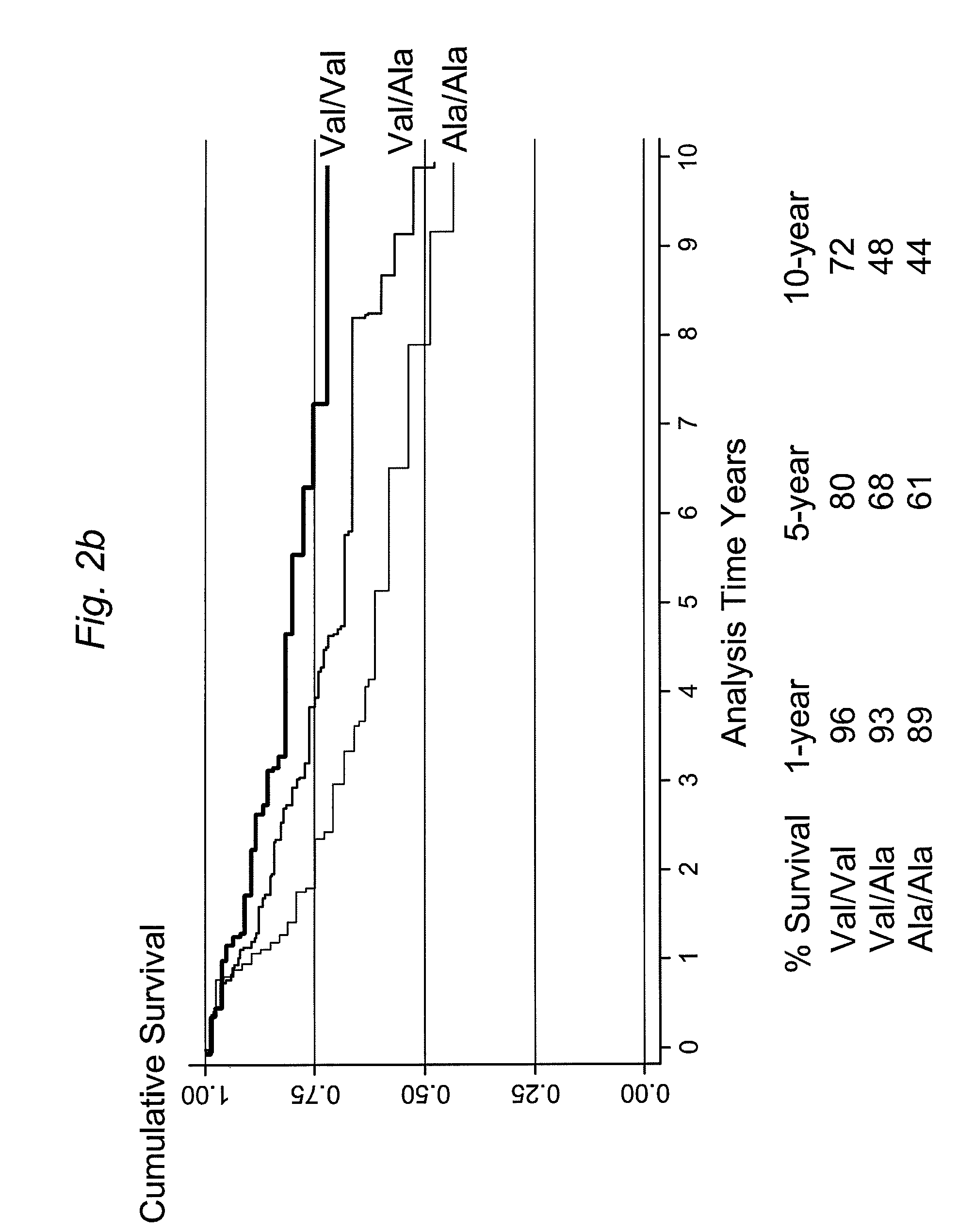
The present invention provides, for the first time, the finding that the manganese superoxide dismutase Val16Ala polymorphism is significantly associated with prognosis for cancer patients treated with chemotherapeutic drug therapy. The alanine allele is a novel biomarker that predicts poor response and poor outcome to chemotherapeutic drug cancer therapy. Conversely, the valine allele predicts a good response and a good outcome to chemotherapeutic drug cancer therapy. Therefore, a genotype assay can be used to determine which alleles a subject is carrying, and subsequently this information can be used to determine if chemotherapeutic drug therapy is appropriate, and to customize therapy according to the patient's MnSOD genotype.
Owner:UNITED STATES OF AMERICA
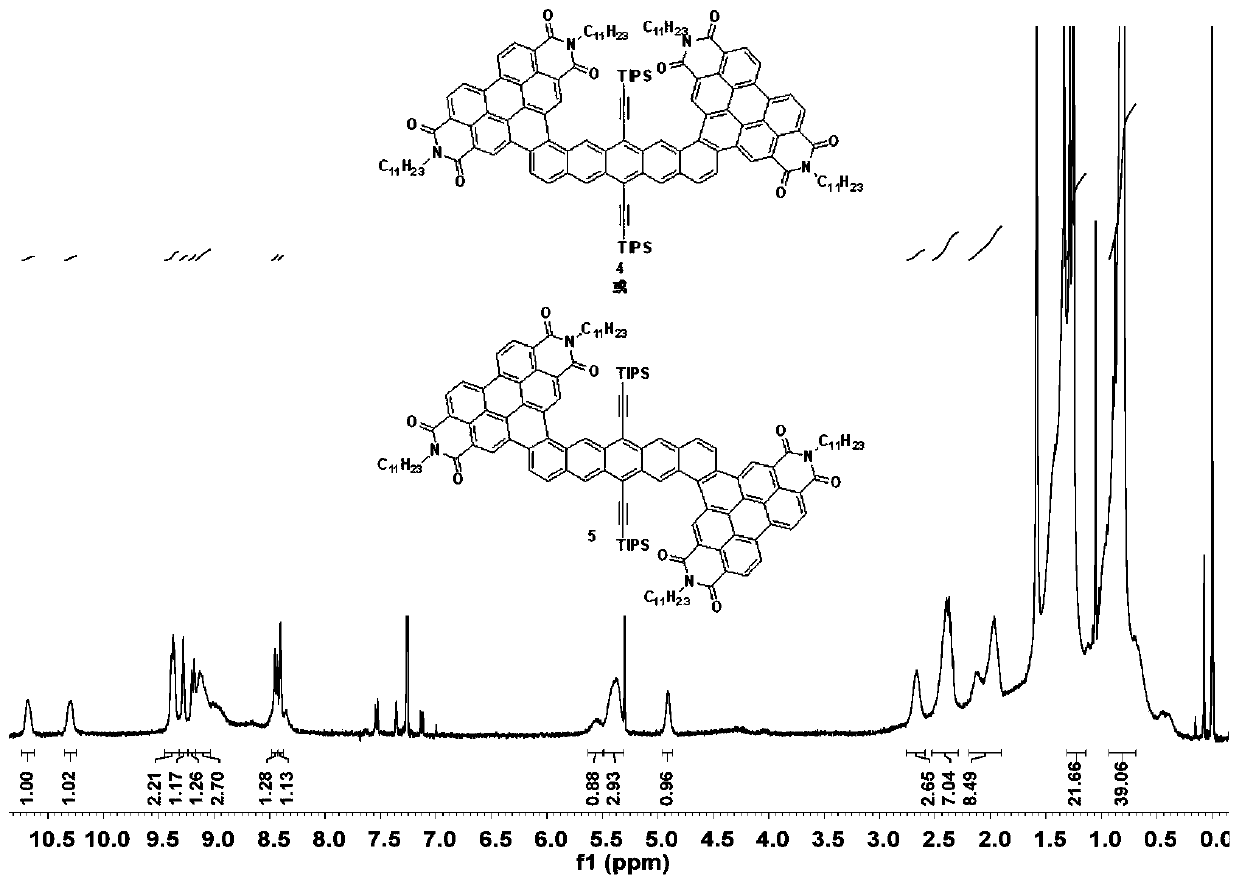
Synthesis method of a-d-a type organic optoelectronic small molecules based on perylene diimide and pentacene
ActiveCN107793444BEasy to prepareQuick spin drySilicon organic compoundsSolid-state devicesOrganic solar cellPtru catalyst
The present invention involves the synthesis method of A‑D‑A organic optoelectronics small molecules based on bonamine and parallel benzene. In an inert atmosphere, 6,13‑ double (silicon aceteraceae)Benzal 2,9频 two -frequency two -frequency alcohol or 6,13‑ double (triangular acenenethyl) and pentazide 2,10‑ binary alcohol and 1‑ bromacide amine dissolved inIn the solvent, under the action of a catalyst, 2,10‑ ‑ two -苝 diimide ‑ 6,13‑ (silicon acetethite group) is obtained through the coupling reaction.Diablytic 1 6,13‑ double (silicon aceteraceae group) and five benzene, then under the action of iodine single, use LED light photos to prepare the final product through a halo reaction.For the first time, the invention has assembled the model molecules of electronic receptor materials and single -line state fission for the first time as a molecule, which can be applied in the fields of organic solar cells, field effect transistors, photoelectric detectors, and organic light diode.
Owner:SHANGHAI NORMAL UNIVERSITY
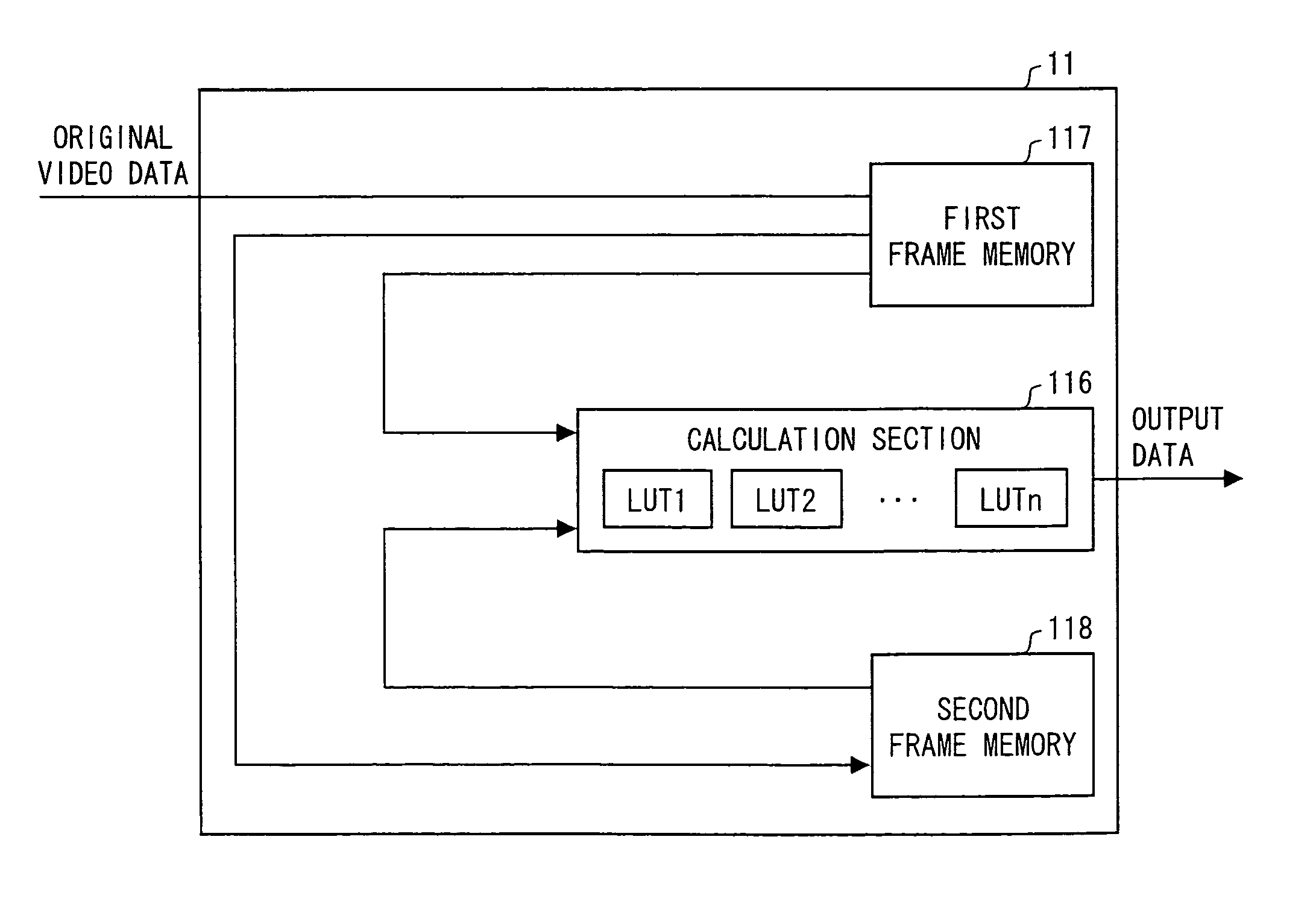
Liquid crystal display devices and methods for driving the same
InactiveUS8760476B2Improve response speedSuppress image blurCathode-ray tube indicatorsInput/output processes for data processingImaging processingLiquid-crystal display
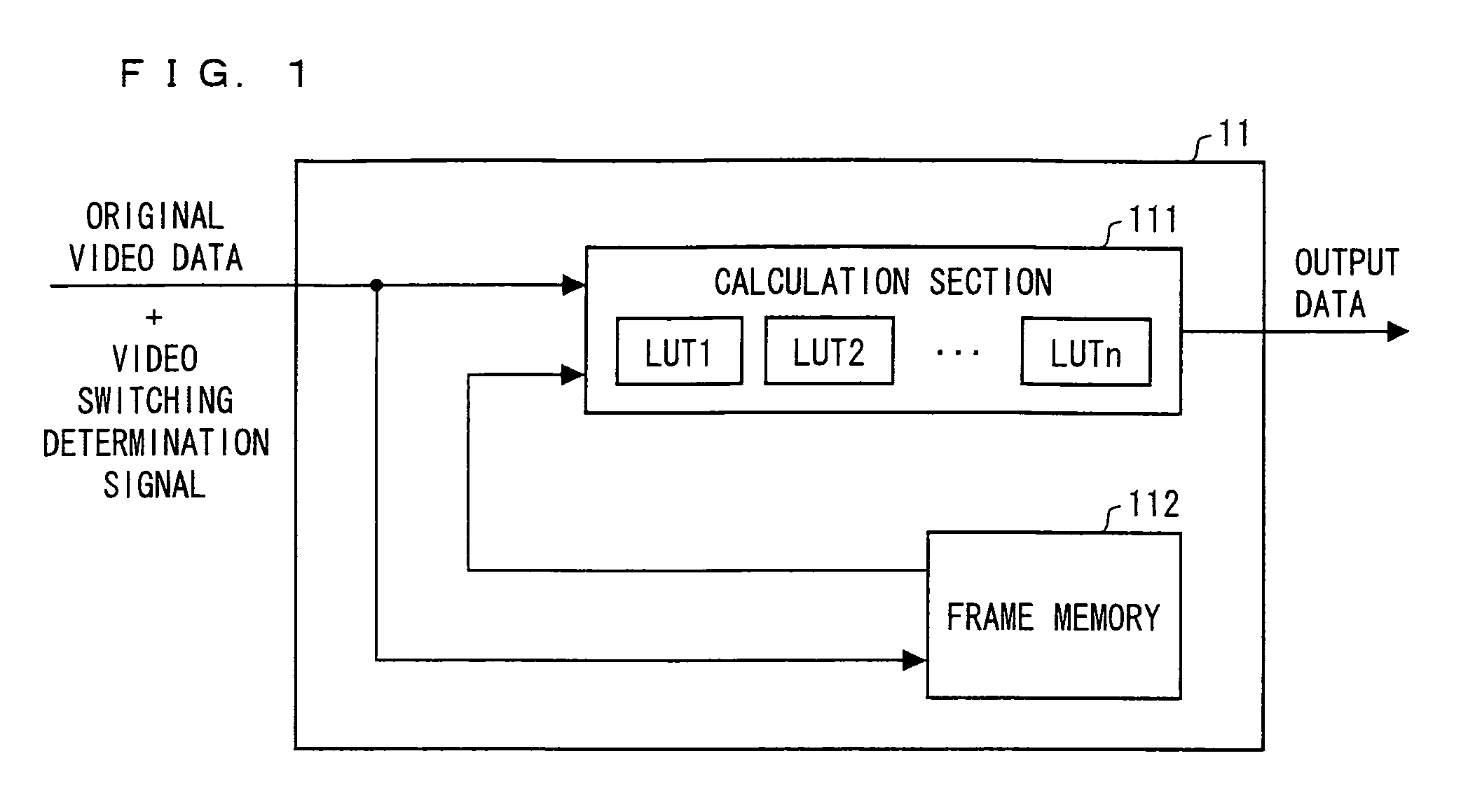
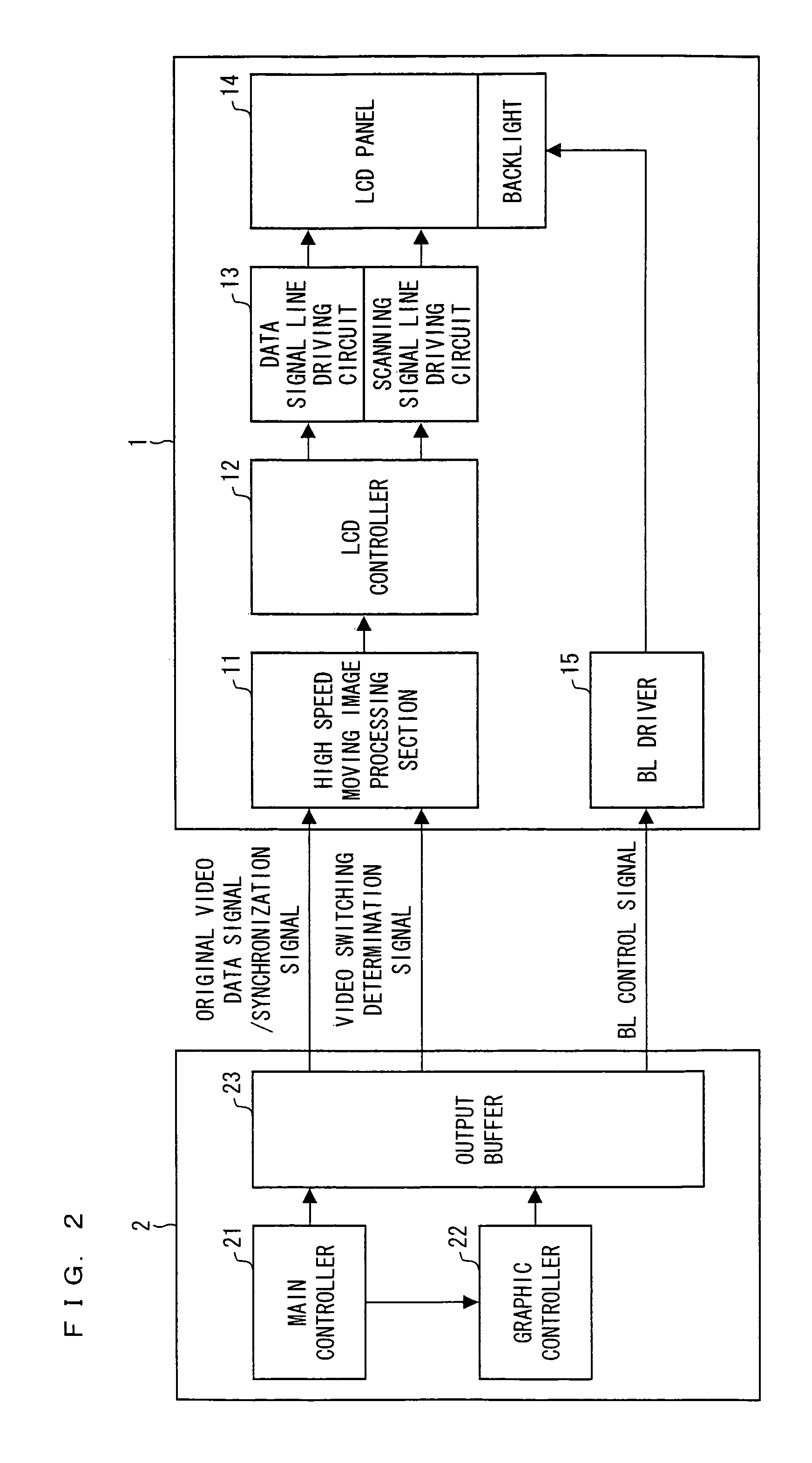
A high speed moving image processing section of a liquid crystal display device includes: a calculation section having a plurality of LUTs in accordance with which an output for performing overshoot drive is obtained with reference to current frame data and previous frame data; and a frame memory in which a video data signal of a previous frame is stored. During each writing period in a single frame period, the calculation section carries out data conversion for performing the overshoot drive by using a video data signal, transmitted from the host device, as current frame data, and by using a video data signal, read out from the frame memory, as pervious frame data. Further, an LUT for performing the overshoot drive is switched in every writing period.
Owner:SHARP KK
Composition and method for improving sleep duration and quality
ActiveUS10376566B2Large dropout ratePoor responsePeptide/protein ingredientsHeavy metal compound active ingredientsSide effectPhysiology
An intervention for sleep disorders comprises: collagen, a gelatin peptide, or the amino acid glycine; L-theanine; lactucopicrin, deoxylactucopicrin, or another lactucopicrin derivative; hyaluronic acid; epigallocatechin gallate; and quinic acid. The intervention helps regulate pro-inflammatory biomarkers that are often associated with insomnia development and progression. These biomarkers include cytokines and enzymes associated with tryptophan degradation. Inhibition of these enzymes and cytokines improves tryptophan availability and sleep quality, and allows individuals to sleep better with fewer side effects. The composition promotes high-quality, deep sleep.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Preparation method and application of medical ultra-high molecular weight poly (p-dioxanone)
The invention discloses a preparation method of medical ultra-high molecular weight poly (p-dioxanone). The preparation method comprises the following steps: 1) purifying p-dioxanone; 2) polymerization reaction: heating a reaction kettle, filling inert gas into the reaction kettle, adding the purified p-dioxanone prepared in the step 1), and adding a catalyst and an alcohol initiator for reaction; (3) after the reaction is finished, taking out the materials and crushing the materials; and 4) heating the crushed material under a vacuum condition to remove unreacted p-dioxanone, and finally preparing the poly (p-dioxanone). The high-molecular-weight poly (p-dioxanone) prepared by the preparation method can meet medical requirements, the molecular weight can reach 300000 to 2000000, and the production magnitude can reach dozens of kilograms by using a catalyst stannous octoate accepted by FDA (Food and Drug Administration).
Owner:苏州西脉红枫生物科技有限公司
Manganese superoxide dismutase val16ala polymorphism predicts resistance to chemotherapeutic drug cancer therapy
InactiveUS20090136952A1Poor responsePoor outcomeMicrobiological testing/measurementBiological testingDrugChemotherapeutic drugs
The present invention provides, for the first time, the finding that the manganese superoxide dismutase Val16Ala polymorphism is significantly associated with prognosis for cancer patients treated with chemotherapeutic drug therapy. The alanine allele is a novel biomarker that predicts poor response and poor outcome to chemotherapeutic drug cancer therapy. Conversely, the valine allele predicts a good response and a good outcome to chemotherapeutic drug cancer therapy. Therefore, a genotype assay can be used to determine which alleles a subject is carrying, and subsequently this information can be used to determine if chemotherapeutic drug therapy is appropriate, and to customize therapy according to the patient's MnSOD genotype.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com