Method of Treating Cancer Using Dithiocarbamate Derivatives
a technology of dithiocarbamate and cancer, applied in the field of cancer treatment, can solve the problems of uncontrolled growth of malignant cells, poor response of other cancers to chemotherapy, death of patients, etc., and achieve the effect of convenient and convenient treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
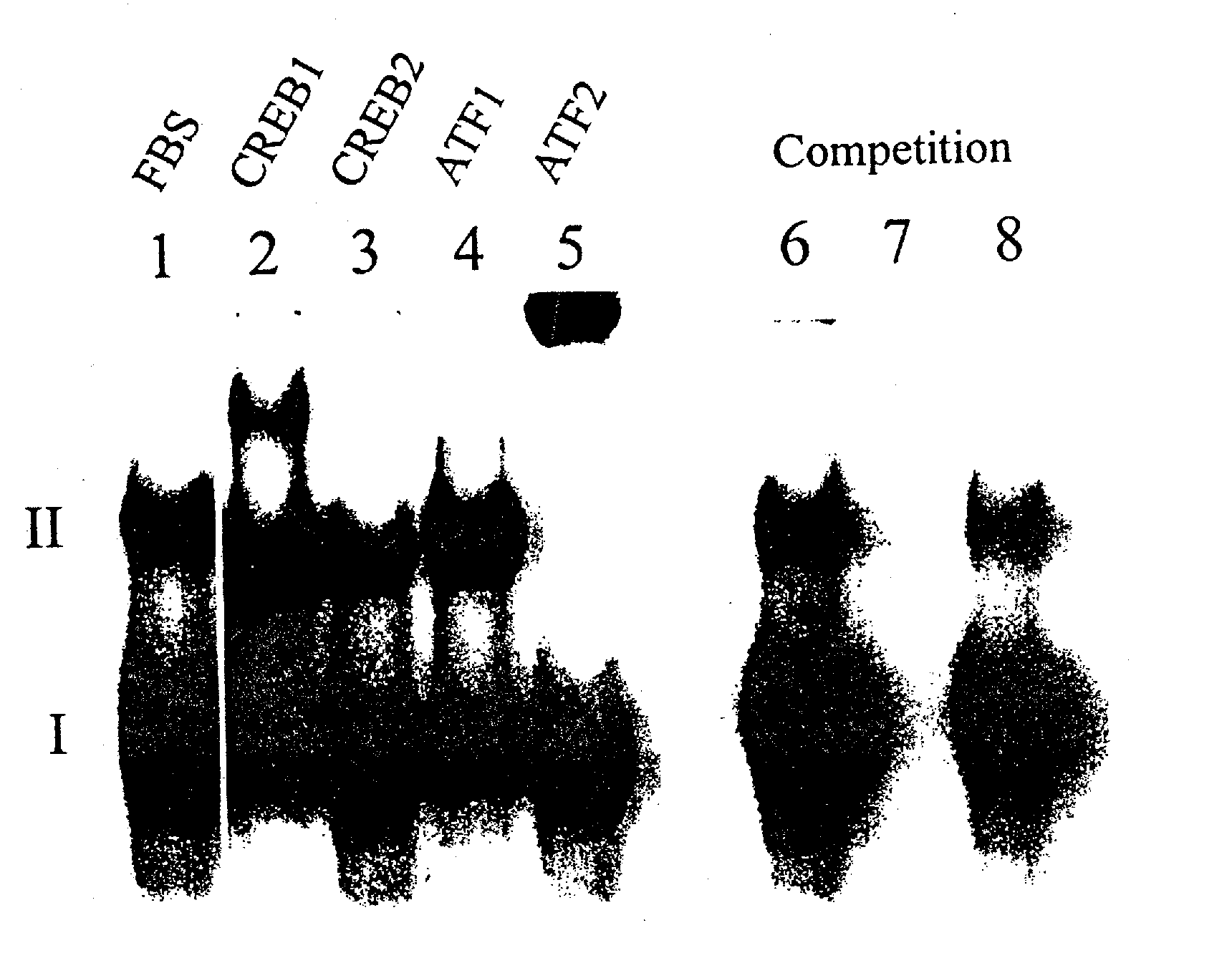
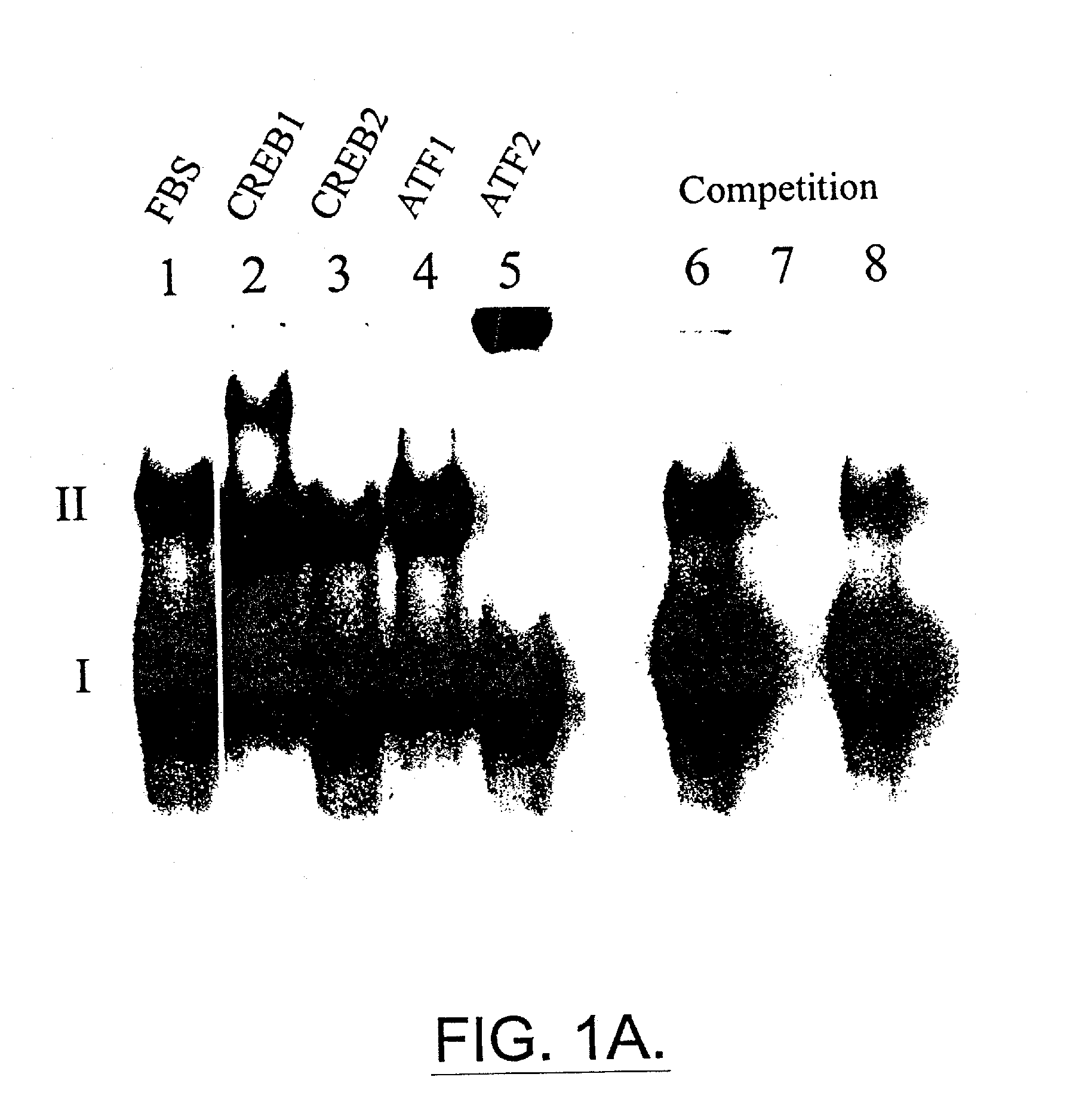
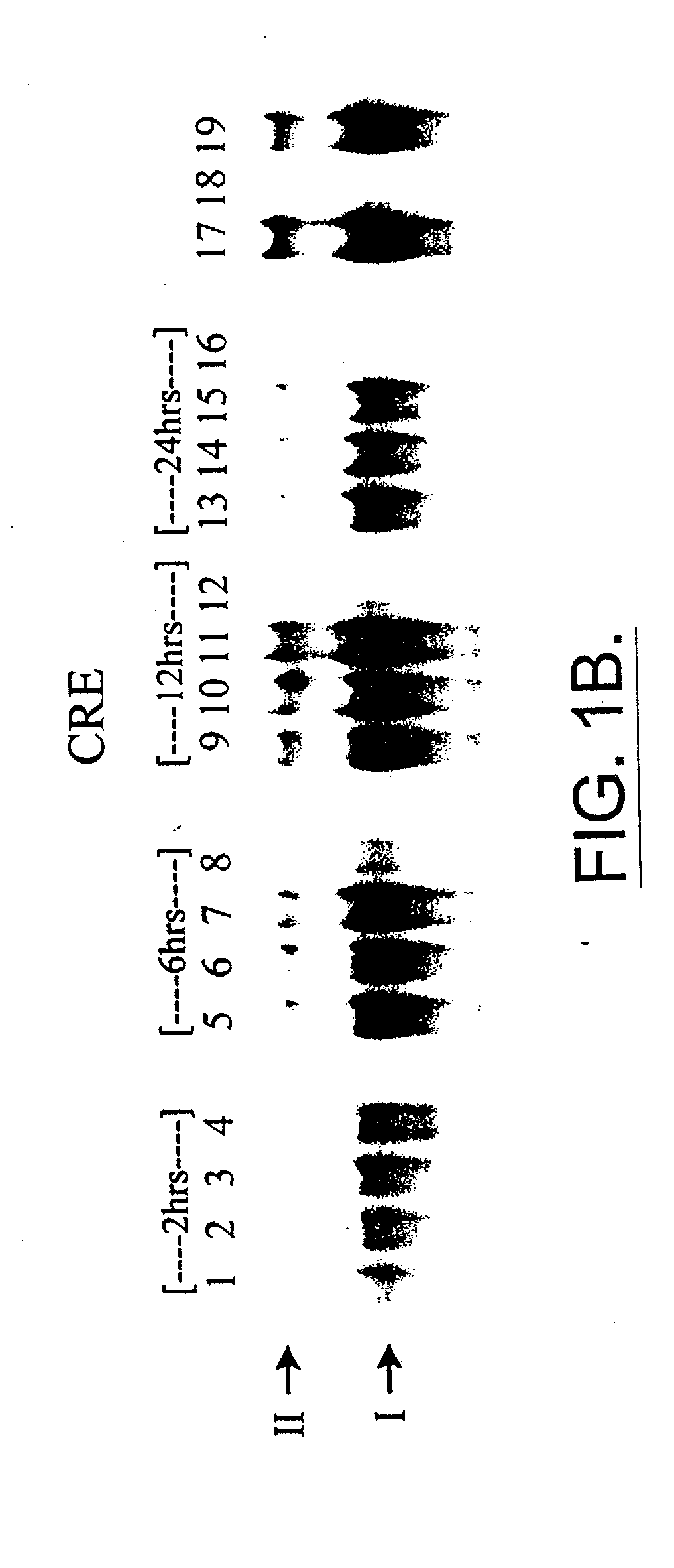
[0115] This example shows dithiocarbamate disulfides inhibit DNA binding to the cyclic AMP response element.
[0116] M1619 melanoma cells were grown to 60% confluence on 100×15 mm plastic Petri dishes, nuclear protein was harvested and electrophoretic mobility gel shift assays (EMSAs) were performed using. The results are shown in FIGS. 1A-1C. Treatment of cells for 6, 12 or 24 hour with the combination of 5 μM disulfuram and 1.6 μM cupric sulfate substantially interrupts transcription factor binding to CRE. EMSAs for 2, 6, 12 or 24 hours of treatment: FBS alone, lanes 1, 5, 9, and 13; FBS+DMSO vehicle, lanes 2, 6, 10, 14; FBS+disulfuram, lanes 3, 7, 11, 15; FBS+disulfuram+CuSO4, lanes 4, 8, 12, 16.
[0117] CRE complexes (I and II) are labeled. Nuclear protein from proliferating M1619 malignant melanoma cells showed two strong constitutive bands (I and II) of DNA binding activity in electrophoretic mobility shift assays with the cyclic AMP response element (CRE) consensus sequence (FI...
example 2
[0124] This example shows that dithiocarbamate disulfides and copper inhibit cyclin A expression. It is known that heterodimers of the transcription factors CREB-1 and c-Fos or ATF2 and Jun family members positively regulate cyclin A expression through binding to a CRE element in the cyclin A promoter.
[0125] Since disulfuram and copper disrupt transcription factor DNA binding to CRE, their effect on expression of cyclin A was studied. FIG. 3A shows disulfuram and copper reduce expression of the cell-cycle protein cyclin A. M1619 melanoma cells were plated at equal densities in 60×15 mm plastic dishes, grown to 80% confluence and treated with DMSO vehicle (5 μl / ml), disulfuram (DS, 5 μM), or the combination of disulfuram and CuSO4 (1.6 μM). After the indicated times, cells were lysed and protein extracts were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) followed by Western blotting using a rabbit polyclonal antibody (Santa Cruz). Typical experiments are shown for 2, 4,...
example 3
[0128] This example illustrates that disulfuram is antiproliferative against melanoma and other tumor cell lines. Disruption of cyclin A expression should impair cell cycle progression and cellular proliferation. Therefore, the effect of disulfuram on M1619 melanoma growth, using concentrations readily achieved in humans on usual clinical doses was studied. Disulfuram was a potent inhibitor of growth in vitro for M1619 melanoma (FIG. 4A). FIG. 4A shows that disulfuram inhibits proliferation of M1619 human melanoma cell lines. Cells stimulated with 10% fetal bovine serum (FBS) were plated at a density of 50,000 cells per well, and DMSO vehicle (5 μl per ml) or disulfuram (DS) was added to wells at the indicated concentrations. After 24 hours, proliferation was quantitated by assessing the cell number-dependent reduction of the soluble yellow tetrazolium dye 3-[4,5-dimethylthiazol]-2-yl-2,5-diphenyl tetrazolium bromide (MTT) to its insoluble formazan, measured as the absorbance at 540...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com