Method for alkaline non-aqueous wet-process catalytic oxidation of hydrogen sulfide
A technology for catalytic oxidation and hydrogen sulfide, applied in chemical instruments and methods, separation methods, gas fuels, etc., can solve problems such as slow regeneration speed, increased operating costs, and equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
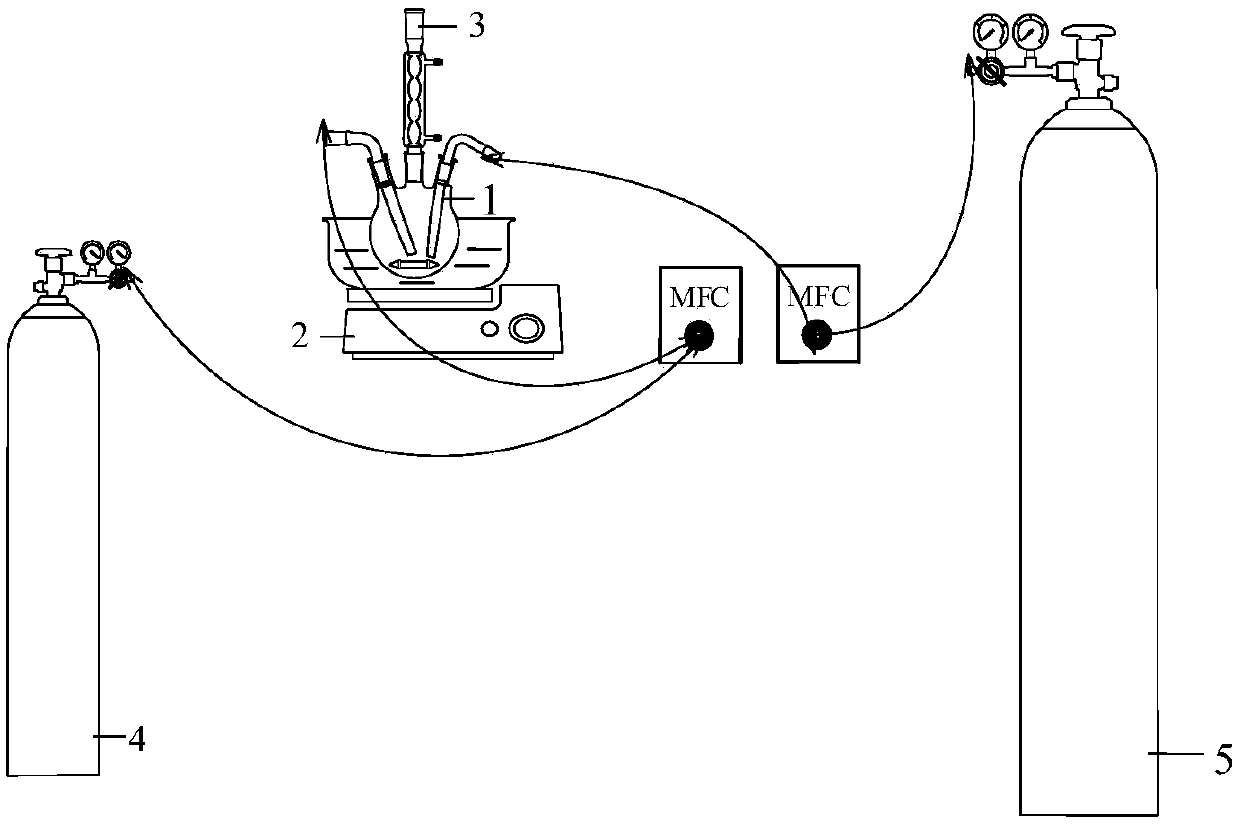
[0026] (1) The gas containing hydrogen sulfide includes natural gas, biogas, refinery tail gas, shale gas and other gases containing hydrogen sulfide, and 0%<volume concentration of hydrogen sulfide≤100%.
[0027] (2) The disubstituted alkylimidazole hydroxide is 1,3-dialkylimidazole hydroxide, 1-alkenyl-3-alkylimidazole hydroxide, 1-benzyl-3-alkylimidazole hydroxide and At least one of 1-benzyl-3-enylimidazoles, wherein the alkyl group contains 1-16 carbons, and the alkene group contains 2-8 carbons. And the pH value of the alkaline ionic liquid is lower than 10.
[0028] (3) The organic solvent with a boiling point higher than 150°C can be N,N-dimethylformamide (DMF), N-dimethylpyrrolidone (NMP), dimethyl sulfoxide (DMSO) in the prior art , at least one of 1,3-dimethyl-2-imidazolidinone (DMI).
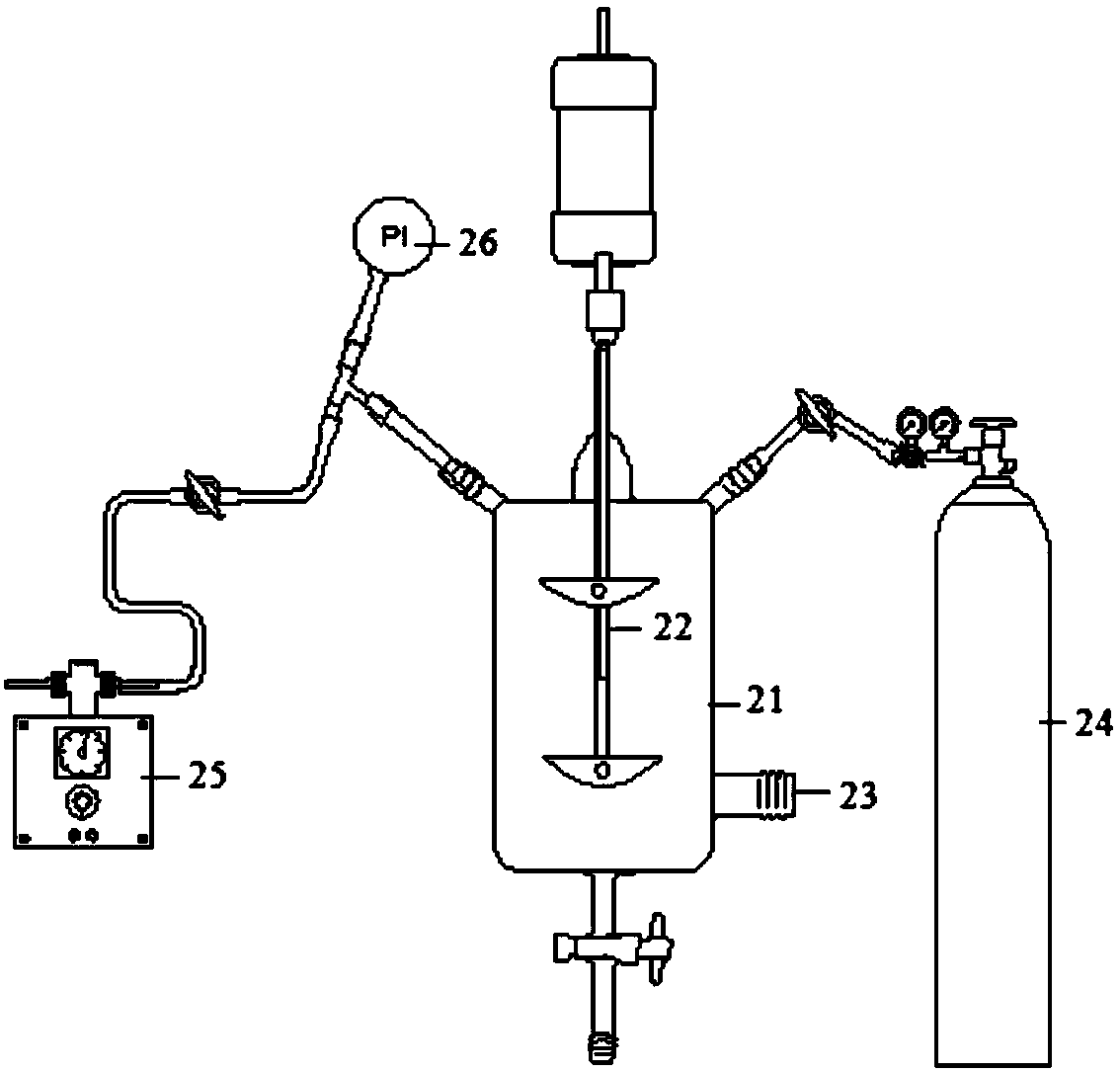
[0029] (4) The use of the iron-based ionic liquid desulfurization solution to carry out the catalytic oxidation treatment of the gas containing hydrogen sulfide can include the fol...
Embodiment 1
[0036] A kind of method of basic non-aqueous phase wet catalytic oxidation hydrogen sulfide, comprises the following steps:
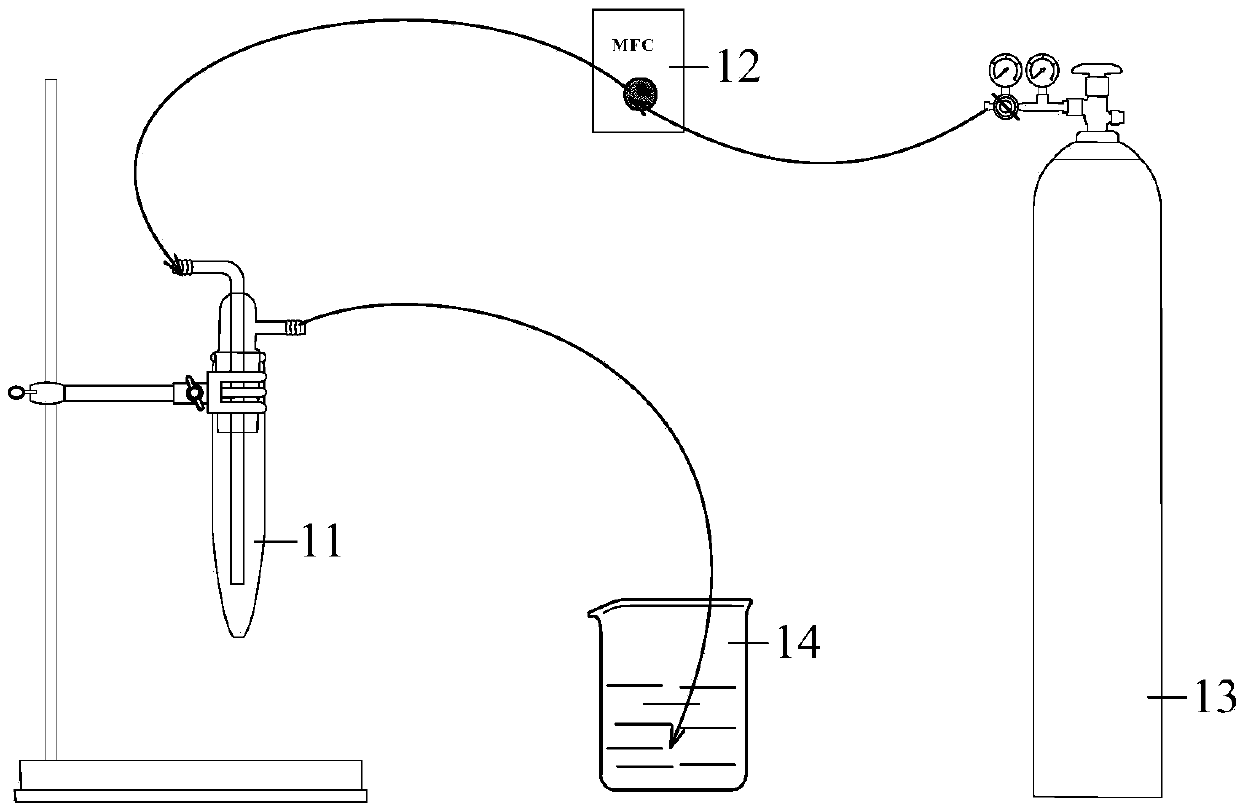
[0037] Step A1. Dissolve 2.10 mol (about 83.9937 g) of sodium hydroxide in 100 mL of deionized water, and dissolve 0.70 mol (about 189.2073 g) of ferric chloride in an appropriate amount of deionized water, then mix the two, fully stir, and Filtration resulted in precipitation of ferric hydroxide. The ferric hydroxide precipitate was washed three times with tap water, and fresh ferric hydroxide solution was obtained after suction filtration.
[0038] Step B1. Add 204.5680g of ethylenediaminetetraacetic acid (H4EDTA) to the ferric hydroxide solution just prepared in step A1, add 300mL of deionized water, heat and boil until the solution is brown and transparent, and then get Iron ethylenediaminetetraacetate (Fe-EDAT) aqueous solution.
[0039] Step C1, dissolve 16.0608g of potassium hydroxide in 80ml of ethanol, and stir to form a suspension, then add ...
Embodiment 2
[0047] A kind of method of basic non-aqueous phase wet catalytic oxidation hydrogen sulfide, comprises the following steps:
[0048] Step A2. Dissolve 2.10 mol (about 83.9937 g) of sodium hydroxide in 100 mL of deionized water, dissolve 0.70 mol (about 189.2073 g) of ferric chloride in an appropriate amount of deionized water, then mix the two, fully stir, and Filtration resulted in precipitation of ferric hydroxide. The ferric hydroxide precipitate was washed three times with tap water, and fresh ferric hydroxide solution was obtained after suction filtration.
[0049] Step B2, add 204.5680g of ethylenediaminetetraacetic acid (H4EDTA) to the ferric hydroxide solution just prepared in step A2, add 300mL of deionized water, heat and boil until the solution is a brown transparent solution, and then obtain after cooling Iron ethylenediaminetetraacetate (Fe-EDAT) aqueous solution.
[0050] Step C2, dissolving 16.0608g of potassium hydroxide in 80ml of ethanol, and stirring to fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com