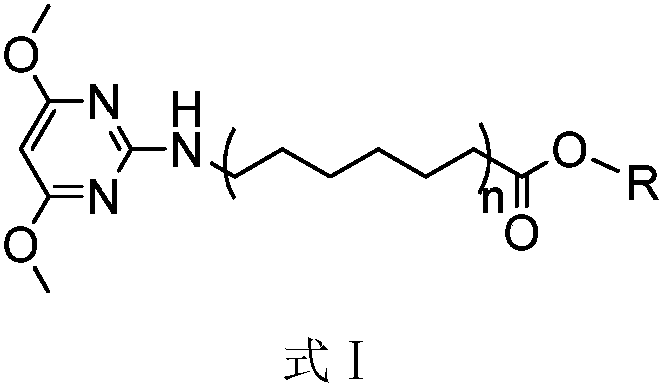
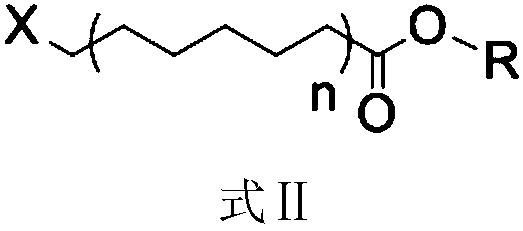
4,6-dimethoxy-2-aminopyrimidine fatty acid derivative as well as preparation method and purpose thereof
A technology of fatty acid derivatives and aminopyrimidine, which is applied in the field of drug synthesis, can solve the problems of non-target organisms and environmental adverse effects, excessive pesticide residues, etc., and achieve good market development prospects, mild reaction conditions, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Ethyl-7-((4,6-dimethoxypyrimidine-2-)aminoheptanoate
[0047]
[0048] Add 155 mg (1 mmol) of 2-amino-4,6-dimethoxypyrimidine solid and 8 mL of dried DMF to a 50 mL pear-shaped bottle, stir to dissolve and add 2 g of anhydrous K 2 CO 3 , stirred at room temperature for 0.5h, then slowly added 194uL (1mmol) of ethyl 7-bromoheptanoate to the reaction system, stirred overnight at room temperature and monitored by TLC until the reaction was complete. After the reaction was completed, 40 mL of water was added thereto, and then extracted with 2×50 mL of ethyl acetate, and the combined organic phases were sequentially washed with 2×50 mL of 1N HCl solution and 2×50 mL of saturated sodium chloride solution. The organic phase was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, then purified on a silica gel column, and eluted with petroleum ether / ethyl acetate (v / v, 6 / 1) to obtain a light yellow oily liquid with a yi...
Embodiment 2
[0050] Synthesis of o-tolyl 5-(4,6-dimethoxypyrimidine-2-)aminovaleric acid
[0051]
[0052] Add 155 mg (1 mmol) of 2-amino-4,6-dimethoxypyrimidine solid and 8 mL of dried DMF to a 50 mL pear-shaped bottle, stir to dissolve and add 2 g of anhydrous K 2 CO 3 , stirred at room temperature for 0.5 h, then slowly added 270 mg (1 mmol) of o-methylphenyl 5-bromopentanoate to the reaction system, stirred overnight at room temperature and monitored by TLC until the reaction was complete. After the reaction was completed, 40 mL of water was added thereto, followed by extraction with 2×50 mL of ethyl acetate, and the combined organic phases were successively washed with 2×50 mL of 1N HCl solution and 2×50 mL of saturated sodium chloride solution. The organic phase was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, then purified on a silica gel column, and eluted with petroleum ether / ethyl acetate (v / v, 4 / 1) to obtain a light yellow oi...
Embodiment 3
[0054] Synthesis of 7-(4,6-dimethoxypyrimidine-2-)aminoheptanoic acid
[0055]
[0056] Weigh 311 mg of ethyl-7((4,6-dimethoxypyrimidine-2-)aminoheptanoate into a 50L pear-shaped bottle, add 10% hydrochloric acid solution and react at 60-80°C for 2-3 hours, and detect the reaction Completely, stop heating, after the reaction solution is cooled to room temperature.Extract 2 times with ethyl acetate, each 50mL, combine organic phase, decompression evaporate solvent, recrystallization in ethanol, yield 92%.1HNMR (CDCl3, 500Hz), δ: 1.30~1.45(m, 4H, CH2), 1.55~1.65(m, 4H, CH2), 2.28(t, J=7.5Hz, 3H, CH3), 3.37(q, J=6.5Hz, 2H, CH2), 3.82(s, 6H, OCH3), 5.36(s, 1H, Ar-H), 7.01(s, 1H, NH), 10.05(s, 1H, COOH).13C NMR(CDCl3, 125Hz) , δ: 24.55, 27.35, 28.64, 31.25, 35.03, 41.32, 54.38, 72.86, 158.09, 175.09, 178.04, .MS (ESI), m / z: 282 ([M+H]-).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com