Method for biomimetic synthesis of nano zirconium dioxide immobilized laccase
A technology of nano-zirconium dioxide and immobilized laccase, which is applied to biochemical equipment and methods, redox enzymes, immobilized on or in inorganic carriers, etc., can solve difficult problems, improve utilization rate, operate Simple process and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
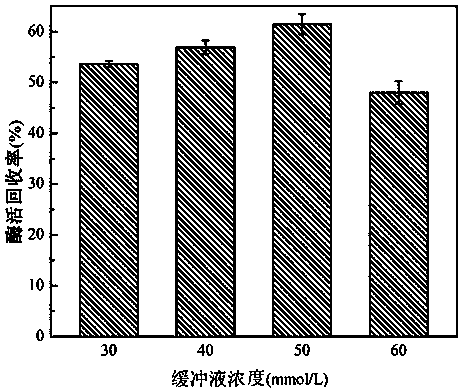
[0032] At room temperature, prepare 5mg / mL lysozyme solution and 0.1U / mL laccase in phosphate buffered saline (PBS) with pH=7.0 and concentrations of 30mmol / L, 40mmol / L, 50mmol / L, and 60mmol / L respectively solution, take 1mL of each of these two solutions and mix well, then add to 8mL 0.1mmol / L K 2 ZrF 6 In the solution, shake gently for 10min, 4000rpm, and centrifuge for 5min to obtain a white precipitate. The resulting precipitate is washed three times with PBS buffer to remove unreacted precursor and unembedded laccase, collect the precipitate, and freeze-dry to obtain nanometer 2 Zirconia immobilized laccase particles.
[0033] The enzyme activity recovery rate of gained immobilized laccase is as follows figure 1 shown. Depend on figure 1 It can be seen that when the concentration of phosphate buffer solution is 50mmol / L, the recovery rate of enzyme activity is the highest.
Embodiment 2
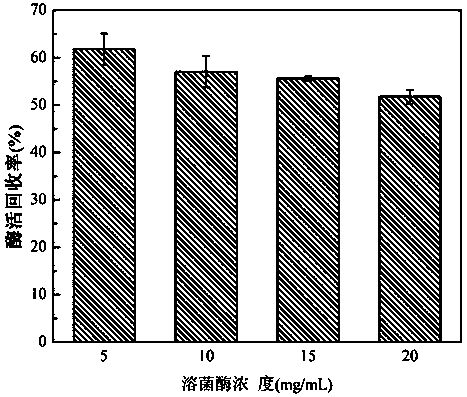
[0035] At room temperature, prepare 5mg / mL, 10mg / mL, 15mg / mL, 20mg / mL lysozyme solutions and 0.1U / mL laccase solutions with pH=7.0, 50mmol / L phosphate buffered saline (PBS), respectively, Take 1mL of lysozyme solutions with different concentrations, mix them with 1mL laccase solution, and add to 8mL 0.1mmol / L K 2 ZrF 6 In the solution, shake gently for 10 minutes, and centrifuge at 4000rpm for 5 minutes to obtain a white precipitate. The obtained precipitate is washed three times with PBS buffer to remove unreacted precursors and unembedded laccase. The precipitate is collected and freeze-dried to obtain nano-dioxide Zirconium-immobilized laccase particles.
[0036] The enzyme activity recovery rate of gained immobilized laccase is as follows figure 2 shown. Depend on figure 2 It can be seen that when the concentration of lysozyme is 5mg / mL, the recovery rate of enzyme activity is the highest.
Embodiment 3
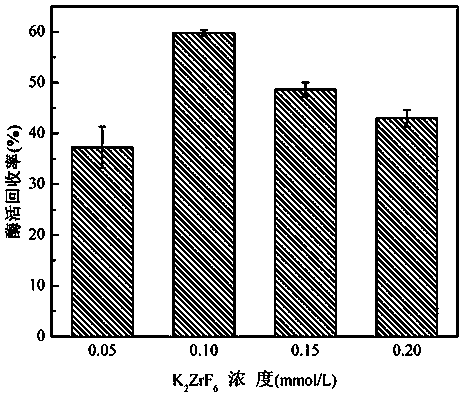
[0038] At room temperature, prepare 5mg / mL lysozyme solution and 0.1U / mL laccase solution with pH=7.0, 50mmol / L phosphate buffered saline (PBS), mix 1mL of each of these two solutions, add To 8mL K 2 ZrF 6In the solution, shake gently for 10 minutes, and centrifuge at 4000rpm for 5 minutes to obtain a white precipitate. The obtained precipitate is washed three times with PBS buffer to remove unreacted precursors and unembedded laccase. The precipitate is collected and freeze-dried to obtain nano-dioxide Zirconium-immobilized laccase particles.
[0039] The enzyme activity recovery rate of gained immobilized laccase is as follows image 3 shown. Depend on image 3 It can be seen that when K 2 ZrF 6 When the concentration of the solution is 0.1mmol / L, the recovery rate of enzyme activity is the highest.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com