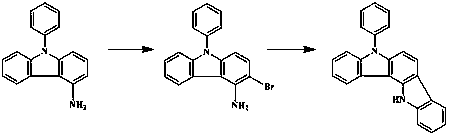
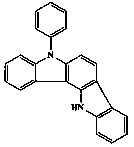
5,12-dihydro-5-phenyl indole (3,2-a)carbazole synthesizing method
A technology of phenylindole and phenylcarbazole, applied in the field of organic chemical synthesis, can solve the problems of difficult removal of isomers, unsuitable operation, high cost, and achieve the effects of easy separation, avoidance of waste and fewer by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Dissolve 51.7 g (0.2 mol) of 4-amino-N-phenylcarbazole in 200 mL of acetonitrile in a 1 L three-necked flask, add 36.4 g (0.18 mol) of 40% hydrobromic acid aqueous solution by mass, and control the reaction temperature- Slowly add 18.4 mL (0.18 mol) of 30% hydrogen peroxide dropwise at 20 °C. After the dropwise addition is complete, the temperature is naturally raised to react overnight. The reaction solution is washed with aqueous sodium bisulfite solution, the organic layer is separated, and hydrochloric acid is added to obtain 3-bromo-4-amino -N-phenylcarbazole hydrochloride, 61.9 g after drying; under the protection of argon, 61.9 g (0.16 mol) 3-bromo-4-amino-N-phenylcarbazole hydrochloride, 33.1 g ortho Bromophenylboronic acid, 20.2 g of cesium carbonate, and 200 mL of N-methylpyrrolidone solvent were added to a 1 L three-necked flask to replace the air system, and then 0.04 g of palladium acetate and 0.16 g of (R,R)-1,2 -bis[(2-methoxyphenyl)phenylphosphino]ethane...
example 2
[0025] In a 2 L three-necked flask, 129.2 g (0.5 mol) of 4-amino-N-phenylcarbazole was dissolved in 1000 mL of acetonitrile, 95.1 g (0.47 mol) of 40% hydrobromic acid aqueous solution was added, and the reaction temperature was controlled at 0 Slowly add 48.0 mL (0.47 mol) of 30% hydrogen peroxide dropwise at ℃. After the dropwise addition is complete, let the temperature rise naturally and react overnight. N-phenylcarbazole hydrochloride, 160.6 g after drying; under argon protection, 160.6 g (0.42 mol) 3-bromo-4-amino-N-phenylcarbazole hydrochloride, 84.3 g o-bromo Add phenylboronic acid, 55.2g cesium carbonate, and 800 mL N-methylpyrrolidone solvent into a 2 L three-neck flask to replace the air system, then add 0.5 g palladium acetate and 1.9 g (R,R)-1,2-bis [(2-Methoxyphenyl) phenylphosphino]ethane, reflux reaction, detection by liquid chromatography, after the end, add 800 mL of ice water to terminate the reaction, extract with 800 mL of dichloromethane, organic layer sod...
example 3
[0027] In a 5 L three-necked flask, 206.7 g (0.8 mol) of 4-amino-N-phenylcarbazole was dissolved in 1600 mL of acetonitrile, and 137.5 g (0.68 mol) of 40% hydrobromic acid aqueous solution was added to control the reaction temperature- Slowly add 89.8mL (0.88mol) of 30% hydrogen peroxide dropwise at 20°C. After the dropwise addition is complete, the temperature is naturally raised to react overnight. The reaction solution is washed with aqueous sodium bisulfite solution, the organic layer is separated, and hydrochloric acid is added to obtain 3-bromo-4-amino -N-phenylcarbazole hydrochloride, 248.1 g after drying; under the protection of argon, 248.1 g (0.66 mol) 3-bromo-4-amino-N-phenylcarbazole hydrochloride, 132.3 g ortho Add bromophenylboronic acid, 71.5 g cesium carbonate, and 1500 mL N-methylpyrrolidone solvent into a 5 L three-necked flask to replace the air system, then add 1.5 g palladium acetate and 6.0 g (R,R)-1,2- Bis[(2-methoxyphenyl)phenylphosphino]ethane, reflux ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com