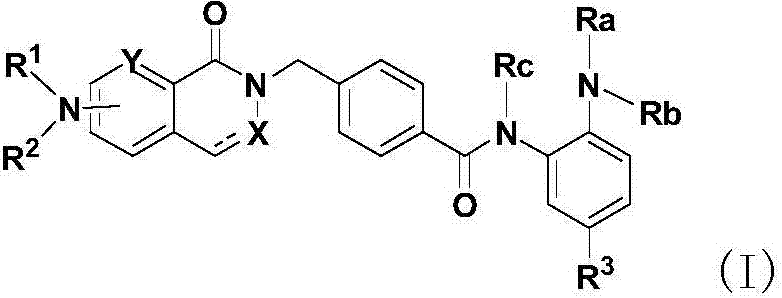
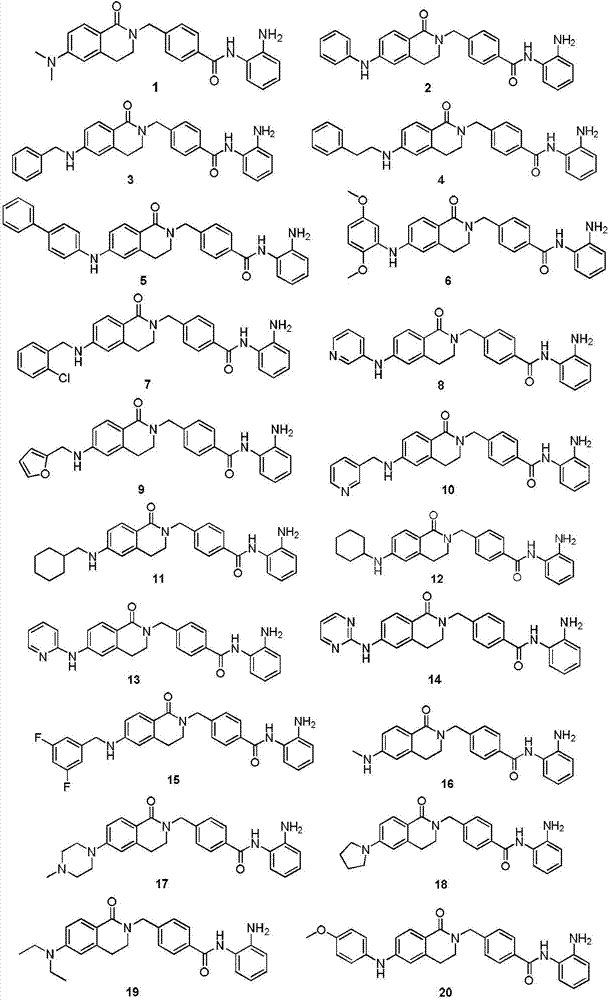
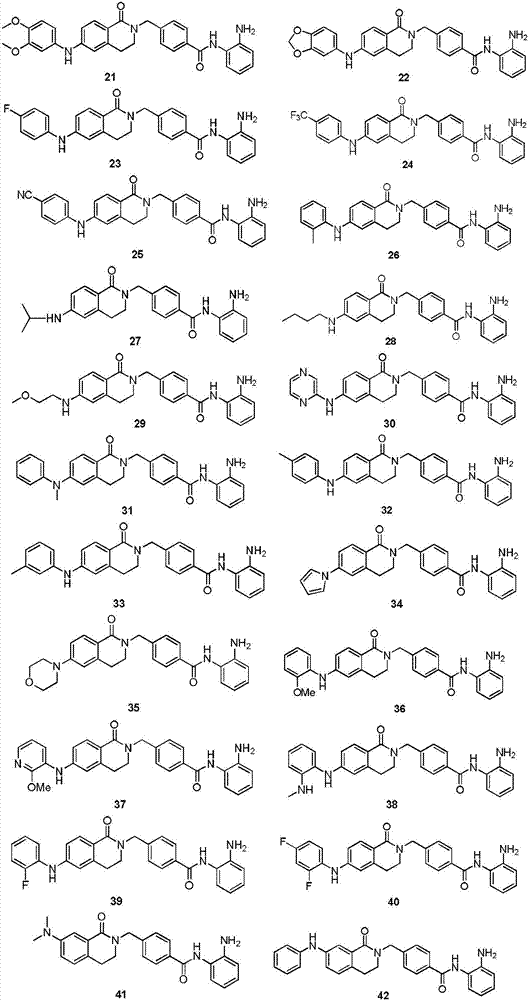
Histone histone deacetylase inhibitor and application thereof
A compound and solvate technology, applied in the field of histone deacetylase inhibitors, can solve problems such as poor metabolic properties and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130]
[0131] synthetic route:
[0132]
[0133] Reagents and conditions: a) 5-bromoindanone, sodium azide, methanesulfonic acid, dichloromethane, 0°C to room temperature; b) methyl 4-bromomethylbenzoate, sodium hydride, N,N-di Methylformamide (DMF), 0°C; c) dimethylamine hydrochloride, cesium carbonate, tris(dibenzylideneacetone) dipalladium (Pd 2 (dba) 3 ), 2-dicyclohexylphosphine-2',4',6'-triisopropylbiphenyl (XPhos), N,N-dimethylformamide, 110°C; d) lithium hydroxide, tetrahydrofuran, water , 100°C; e) o-phenylenediamine, N,N-diisopropylethylamine (DIPEA), O-benzotriazole-N,N,N',N'-tetramethylurea tetrafluoroboric acid (TBTU), N,N-Dimethylformamide, room temperature.
[0134]a) 5-bromoindanone (1.08g, 5.12mmol) was dissolved in 50mL of dichloromethane, methanesulfonic acid (3.32mL, 51.2mmol) was slowly added at 0°C, and then azidation was slowly added in batches to the reaction system Sodium (0.665g, 10.23mmol), slowly warmed up to room temperature, stirred for...
Embodiment 44
[0151]
[0152] synthetic route:
[0153]
[0154] Reagents and conditions: Reagents and conditions: a) 5-bromoindanone, sodium azide, methanesulfonic acid, dichloromethane, 0°C to room temperature; b) methyl 4-bromomethylbenzoate, sodium hydride, N , N-dimethylformamide (DMF), 0°C; c) tert-butyl carbamate, N,N-dimethylethylenediamine, cuprous iodide, potassium carbonate, toluene, 110°C; 20% Tris Dichloromethane solution of fluoroacetic acid, dichloromethane, 0°C; d) thiophene-2-sulfonyl chloride, pyridine, dichloromethane, 0°C; e) lithium hydroxide, tetrahydrofuran, water, 100°C; f) o-benzene Diamine, N,N-diisopropylethylamine (DIPEA), O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid (TBTU), N,N- Dimethylformamide, room temperature.
[0155] c) Add compound C (0.5g, 1.336mmol), tert-butyl carbamate (0.235g, 2.004mmoL), N,N-dimethylethylenediamine (0.014mL, 0.134mmoL) to 10mL toluene under the protection of argon ), potassium carbonate (0.369g, 2.67m...
Embodiment 49
[0162]
[0163] synthetic route:
[0164]
[0165] Reagents and conditions: a) 5-bromoindanone, sodium azide, methanesulfonic acid, dichloromethane, 0°C to room temperature; b) methyl 4-bromomethylbenzoate, sodium hydride, N,N-di Methylformamide (DMF), 0°C; c) dimethylamine hydrochloride, cesium carbonate, tris(dibenzylideneacetone) dipalladium (Pd 2 (dba) 3 ), 2-dicyclohexylphosphine-2',4',6'-triisopropylbiphenyl (XPhos), N,N-dimethylformamide, 110°C; d) lithium hydroxide, tetrahydrofuran, water , 100°C; e) tert-butyl-(4-fluoro-2-nitrophenyl)-carbamate, sodium dithionite, sodium carbonate, tetrahydrofuran, water, 80°C; f) intermediate G, N, N-diisopropylethylamine (DIPEA), O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid (TBTU), N,N-dimethylformamide , room temperature; g) 20% trifluoroacetic acid in dichloromethane, dichloromethane, 0°C.
[0166] e) Compound F (3.2g, 12.49mmol) was dissolved in 40mL of tetrahydrofuran, sodium dithionite (10g, 57.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com