Biological preparing method of key chiral intermediate of levonorgestrel
A technology for a chiral intermediate, levonorgestrel, is applied in the field of preparing a key chiral intermediate of levonorgestrel, can solve the problems of catalyst pollution, unsatisfactory stereoselectivity and the like, and achieves high substrate concentration, high Good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Screening of microbial strains for catalytic reduction of 2,6-dichloro-3-fluorophenethyl alcohol
[0038] 1. Strain screening
[0039] Enrichment culture: Add 1g of fresh soil samples (collected from the campus of Zhejiang University of Technology (Hangzhou, Zhejiang)) into a 250mL shake flask containing 50mL enrichment medium, culture at 30°C and 200rpm for 5 days, and take 1mL for culture The culture medium was transferred to fresh enrichment medium, and the culture was continued for 5 days, and the enrichment was repeated twice. The enrichment medium consists of: (NH 4 ) 2 SO 4 2g / L, KH 2 PO 4 2g / L, NaCl 1g / L, MgSO 4 ·7H 2 O 0.5g / L, the compound of formula (II) (2g / L) is the only carbon source, the solvent is distilled water, pH6.5.
[0040] Plate primary screening: the enriched culture solution was diluted 10 with normal saline 4 -10 6 After doubling, it was spread on the plate to select medium, and cultured at 30°C for 2 days. Pick a single ...
Embodiment 2
[0058] Embodiment 2: Catalyst preparation for bioreduction of ethyl condensates
[0059]1) Slant culture: Inoculate Geotrichum candidum ZJPH1704 into the slant medium, culture at 30°C for 1-2 days, and store in a refrigerator at 4°C. Slant medium formula: glucose 15g / L, peptone 7.5g / L, yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl 0.75g / L, MgSO 4 ·7H 2 O 0.75g / L, agar 20g / L, solvent is distilled water, pH 6.5.
[0060] 2) Seed culture: pick a ring of bacteria from the mature slant and insert it into a 250 ml shake flask containing 100 ml of seed medium, culture at 30° C. and 200 rpm for 12 hours to obtain seed liquid. Seed medium formula: glucose 15g / L, peptone 20g / L, yeast extract 10g / L, (NH 4 ) 2 SO 4 2g / L, KH 2 PO 4 2g / L, NaCl 1g / L, MgSO 4 ·7H 2 O0.5g / L, solvent is distilled water, pH 6.5.
[0061] 3) Fermentation culture: transfer the seed liquid to a 250ml shake flask equipped with 70ml fermentation medium with an inoculum size of 7% i...
Embodiment 3
[0062] Example 3 Bioreduction of Geotrichum candidum ZJPH1704 to prepare ethyl hydroxylate
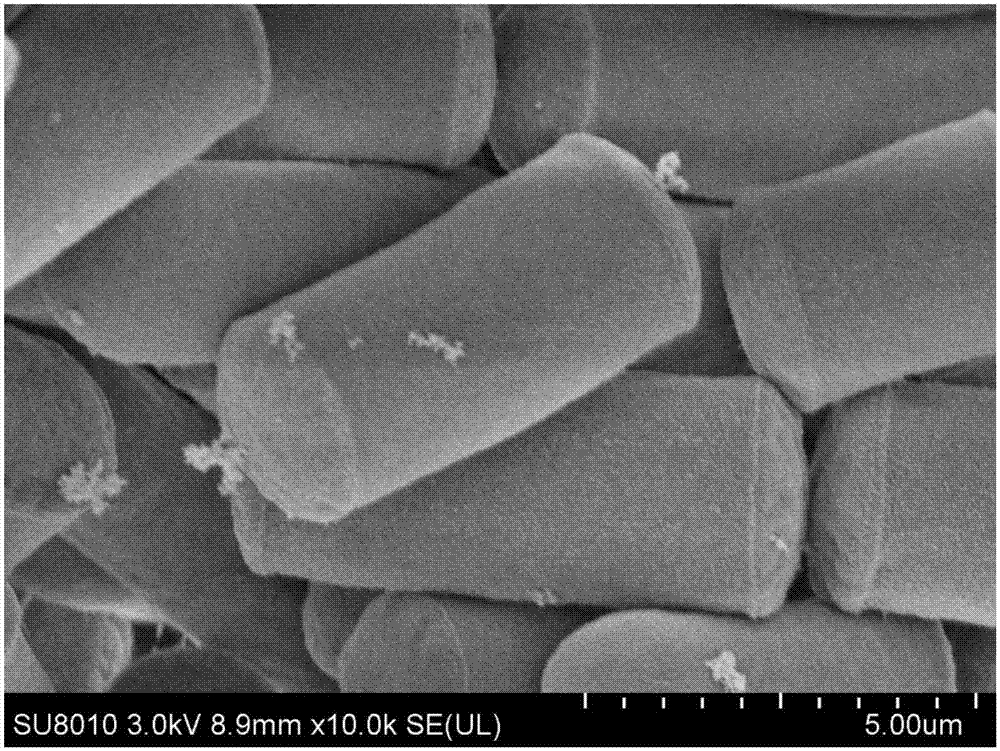
[0063] 3g of Geotrichum candidum ZJPH1704 wet thallus obtained by the method of Example 2 was suspended in 10ml of 0.1M, pH 6.5 phosphate buffer, the final concentration of the wet thallus was 300g / L by wet weight, and a final concentration of 7.5g was added / L The ethyl condensate represented by formula (Ⅲ) is used as the substrate, cultured with shaking at 30°C and 200rpm for 72 hours, tracked and detected by thin-layer chromatography, centrifuged the reaction solution after the reaction, took the supernatant, and added an equal volume of ethyl acetate The ester was extracted twice, and the extracts were combined, and the solvent was evaporated with a rotary evaporator. Gained concentrate is dissolved in anhydrous methanol and then detected by HPLC, see Figure 10 . Simultaneous detection of the substrate ethyl condensate III ( Figure 8 ) and product ethyl hydroxylate IV standard...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com