Calcium and zinc gluconate oral liquid and preparation method thereof
A technology of calcium zinc gluconate and oral solution, applied in the field of medicine, can solve the problems of insufficient stability, easy crystallization and precipitation, high viscosity of the solution, etc., so as to reduce the influence of human blood sugar concentration, reduce safety risks, and achieve fast filtration speed. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
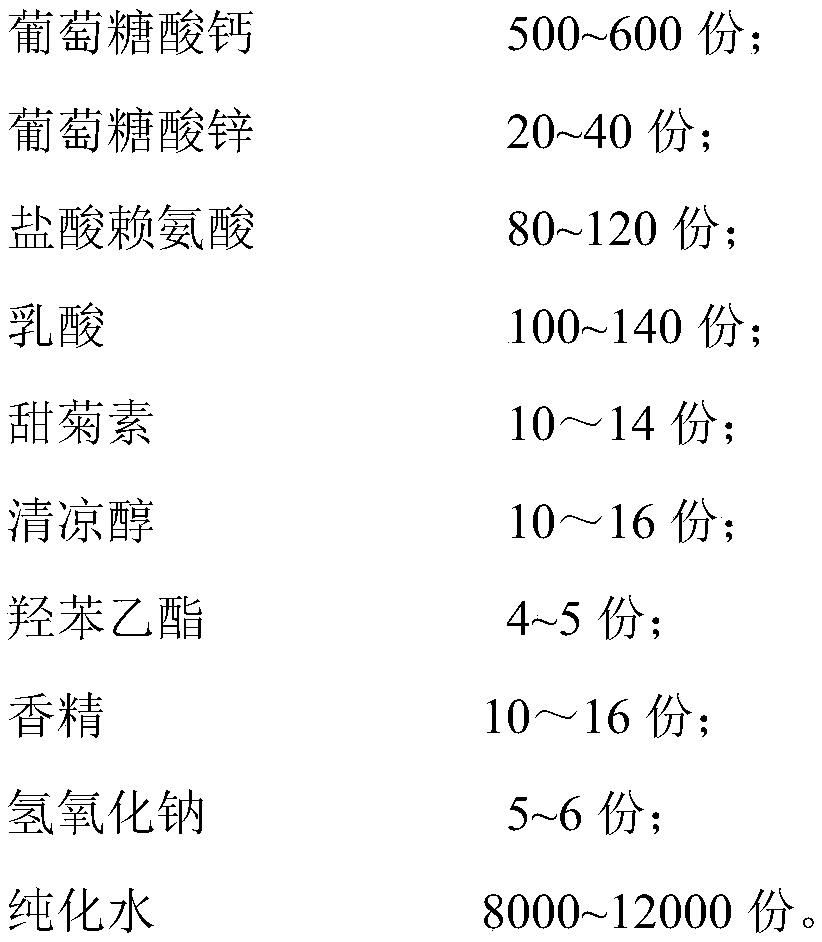
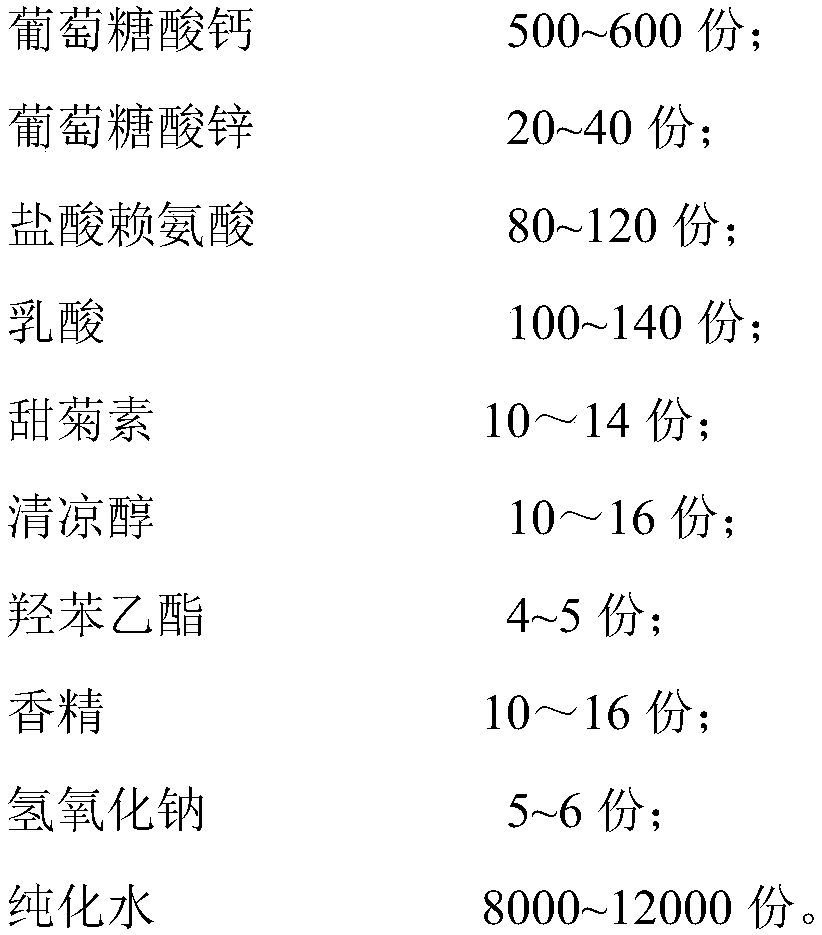
[0027] A calcium zinc gluconate oral solution comprises the following components by weight:
[0028]
[0029] The preparation method of above-mentioned calcium zinc gluconate oral solution comprises the following steps:
[0030] (1) Add calcium gluconate after boiling 8L of purified water for 20 minutes, and keep boiling for 10 minutes to obtain reagent 1;
[0031] (2) Add zinc gluconate, lysine hydrochloride, stevioside and penetrant alcohol to the reagent 1 obtained in step (1), and keep the reagent 2 obtained by boiling for 40 minutes;
[0032] (3) Add ethylparaben to the reagent 2 obtained in step (2), keep boiling for 5 minutes, and cool to 40° C. to obtain reagent 3;
[0033] (4) Add lactic acid and essence to the reagent 3 obtained in step (3), then add the remaining purified water, and adjust the pH to 4.0-5.0 with sodium hydroxide to obtain the reagent 4;
[0034] (5) Filter the reagent 4 obtained in step (4) successively with 0.8 μm and 0.45 μm microporous membr...
Embodiment 2~4
[0061] The formula components and preparation methods of Examples 2-4 are the same as those of Example 1, except that the amounts of stevioside, ethylparaben and essence are different, see Table 6 for the specific amounts.
[0062] The calcium zinc gluconate oral solution obtained by using the formulations in Examples 1-4 was tested for mouthfeel and aroma, and the results are shown in Table 6 below.
[0063] Table 6: Experimental results (parts by weight) of different amounts of stevia, ethylparaben and essence
[0064]
[0065] In the above table, flavors include but not limited to apple flavor and sweet orange juice flavor. From the experimental results in the above table, it can be seen that the weight ratio of stevioside, essence and relieving alcohol is 12:12:14 and the experimental effect is the best.
Embodiment 5
[0067] The formulation components and preparation method of Example 5 are the same as those of Example 1, except that the amount of ethylparaben is different, see Table 7 for the specific amount.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com