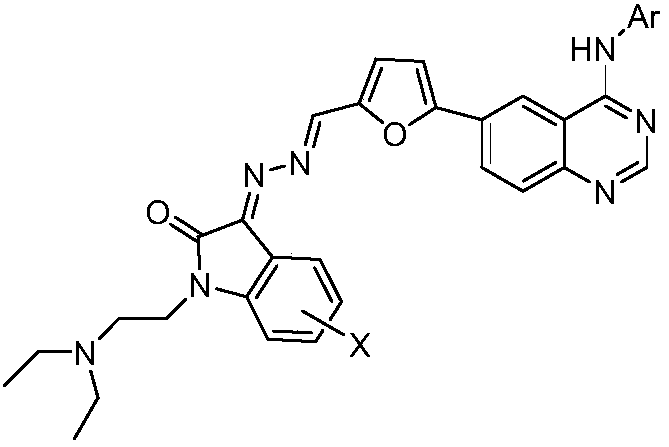
Isatin derivative synthesized by N-substituted isatin hybrid quinazoline compound and application of the isatin derivative in preparation of anti-tumor drugs
A technology of hybrid quinazoline and anti-tumor drugs, which is applied in the field of preparation of anti-tumor drugs, can solve the problems of treatment impact, reduce tumor effect, etc., and achieve the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
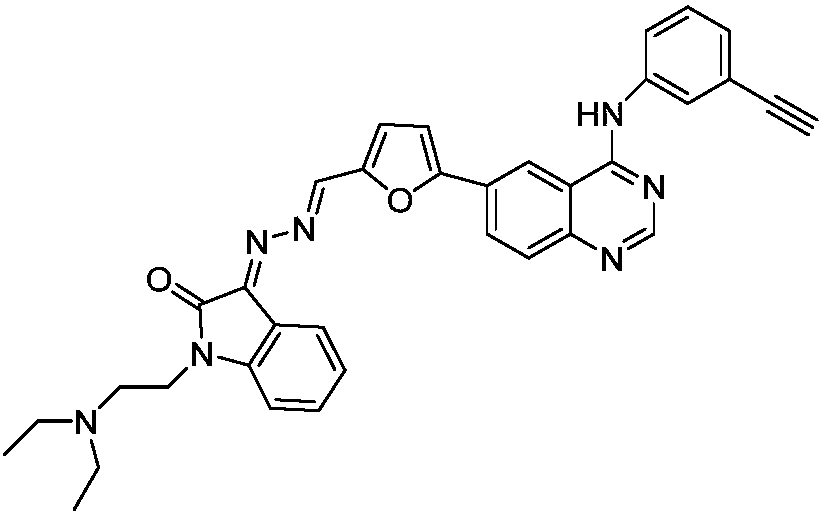
Embodiment 1
[0038] Synthetic compound A
[0039]
[0040] 0.1018g (0.3mmol) 4-(3-ethynylanilino)-6-(5-formylfuran-2-yl)quinazoline, 0.0780g (0.3mmol) (Z)-1-(diethyl Aminoethyl)-3-hydrazone indolin-2-one, 0.3mL acetic acid, 7mL ethanol and 0.1mL N,N-dimethylformamide were added to the reaction flask, and the reaction was refluxed at 79°C for 6 hours. The reaction was completed After cooling to room temperature, the solvent was removed by rotary evaporation under reduced pressure. The residue obtained was recrystallized with MeOH / THF to obtain 0.1361 g of compound A as a red solid, with a yield of 77.9%, mp 242.0-244.4°C, and structural characterization data: HRMS (C 35 H 31 N 7 O 2 )m / z[M+H] + : 582.2614 (calculated value 582.2617); 1 H NMR(600MHz, DMSO-d 6 )δ(ppm): 10.16(s,1H),9.05(s,1H),8.74(s,1H),8.69(s,1H),8.48(d,J=6.4Hz,1H),8.36(d, J = 8.2Hz, 1H), 8.09 (s, 1H), 8.03 (d, J = 7.7 Hz, 1H), 7.93 (d, J = 8.9 Hz, 1H), 7.66-7.65 (m, 1H), 7.54 7.49(m,2H),7.47(t,J=7.7Hz,1H),7.29(d,J=7.3Hz,1H),7...
Embodiment 2
[0042] Synthetic compound B
[0043]
[0044] In this example, the equimolar (Z)-1-(diethylaminoethyl)-3-hydrazone-5-fluoroindolin-2-one is used to replace (Z)-1-(二) in Example 1. Ethylaminoethyl)-3-hydrazone indolin-2-one, other steps are the same as in Example 1, to obtain 0.1340 g of compound B as a red solid, with a yield of 74.6%, mp 253.5-256.2°C, structural characterization data For: HRMS(C 35 H 30 FN 7 O 2 )m / z[M+H] + : 600.2521 (calculated value 600.2523); 1 H NMR(600MHz, DMSO-d 6 )δ(ppm): 10.11(s,1H),9.08(s,1H),8.77(s,1H),8.67(s,1H),8.32(d,J=8.6Hz,1H), 8.24(dd, J = 8.0, 2.0 Hz, 1H), 8.08 (s, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 8.6 Hz, 1H), 7.68 (d, J = 3.5 Hz, 1H), 7.50 (d, J = 3.5 Hz, 1H), 7.45 (t, J = 7.9 Hz, 1H), 7.41 (td, J = 8.7 Hz, 2.7 Hz, 1H), 7.29-7.28 (m, 2H) ,4.21(s,1H),4.14(brs,2H),3.41(brs,2H),3.29-3.22(m,4H),1.22(t,J=6.7Hz,6H); 13 C NMR(151MHz, DMSO-d 6 )δ(ppm): 164.0, 159.0, 157.8, 157.4, 157.3, 154.1(d, 1 J C-F =270Hz),150.8,149.9,149.1,140.6,1...
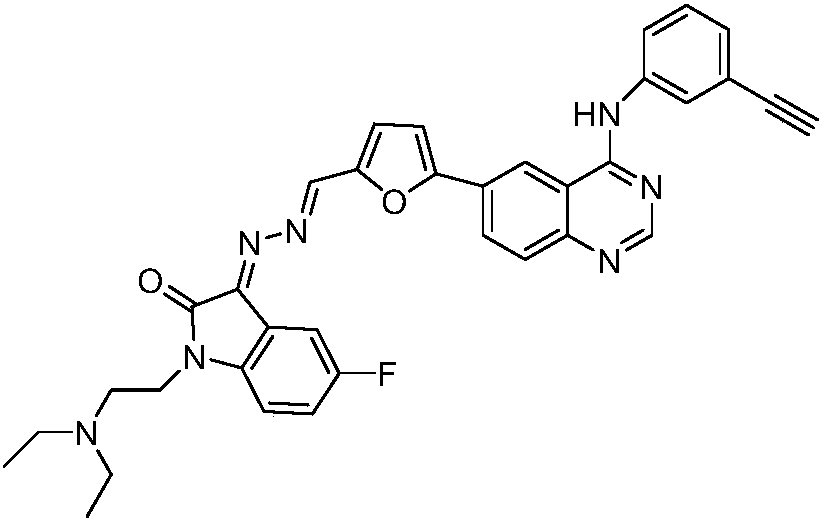
Embodiment 3
[0046] Synthetic compound C
[0047]
[0048] In this example, the equimolar (Z)-1-(diethylaminoethyl)-3-hydrazone-5-chloroindolin-2-one is used to replace (Z)-1-(二) in Example 1. Ethylaminoethyl)-3-hydrazone indolin-2-one, other steps are the same as in Example 1, to obtain 0.1409g of compound C as a red solid, the yield is 76.2%, mp278.7-281.0℃, structural characterization data For: HRMS(C 35 H 30 ClN 7 O 2 )m / z[M+H] + : 616.2218 (calculated value: 616.2228); 1 H NMR(600MHz, DMSO-d 6 )δ(ppm): 10.13(s,1H),9.09(s,1H),8.80(s,1H),8.67(s,1H),8.55(brs,1H),8.37(d,J=8.6Hz, 1H), 8.03 (s, 1H), 7.91 (d, J = 8.2 Hz, 2H), 7.65 (d, J = 3.1 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 7.50 (d, J = 3.1Hz, 1H), 7.45 (t, J = 8.0Hz, 1H), 7.31-7.27 (m, 2H), 4.23 (s, 1H), 4.14 (brs, 2H), 3.40 (brs, 2H), 3.26(brs,4H),1.21-1.20(m,6H); 13 C NMR(151MHz, DMSO-d 6 )δ(ppm): 163.7, 157.8, 157.2, 155.0, 153.2, 150.8, 148.9, 142.9, 139.1, 132.6, 129.0, 128.9, 128.6, 127.1, 126.6, 125.4, 124.3, 123.1, 121.8, 119.6, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com