A kind of preparation method of 4-bromo-1-methyl-2-(trifluoromethyl)benzene
A technology of aminotrifluorotoluene and trifluoromethyl, which is applied in the field of preparation of 4-bromo-1-methyl-2-benzene, can solve the problems such as the inability to meet the high-purity requirements of pharmaceutical intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
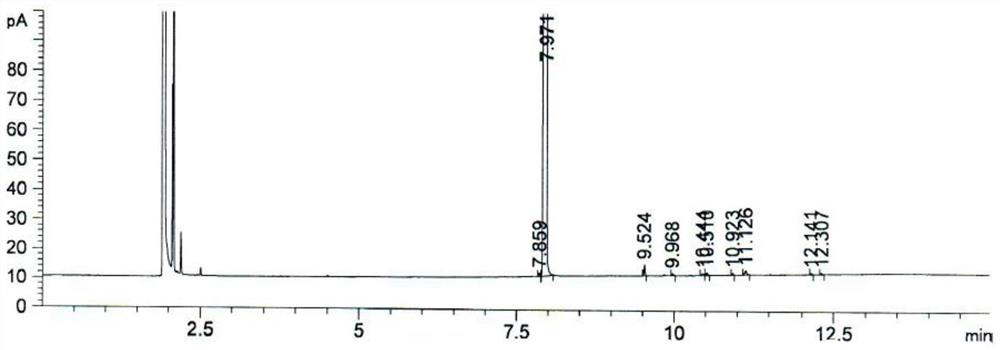
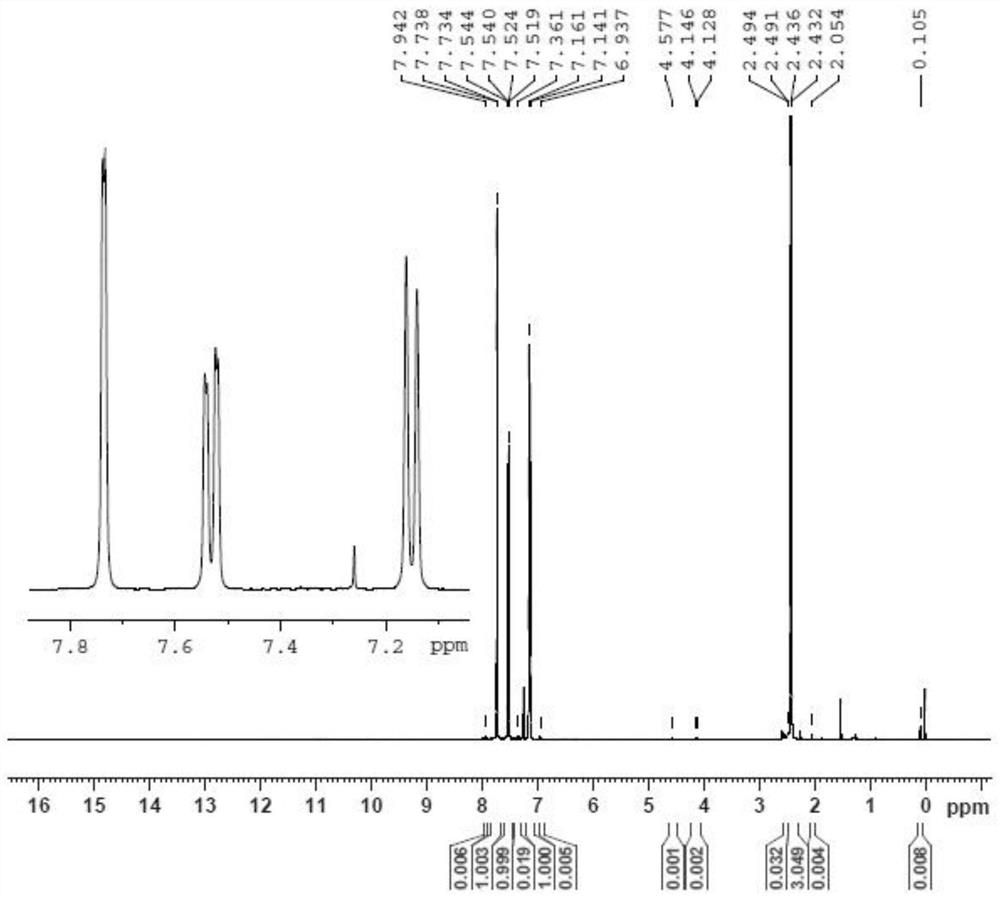
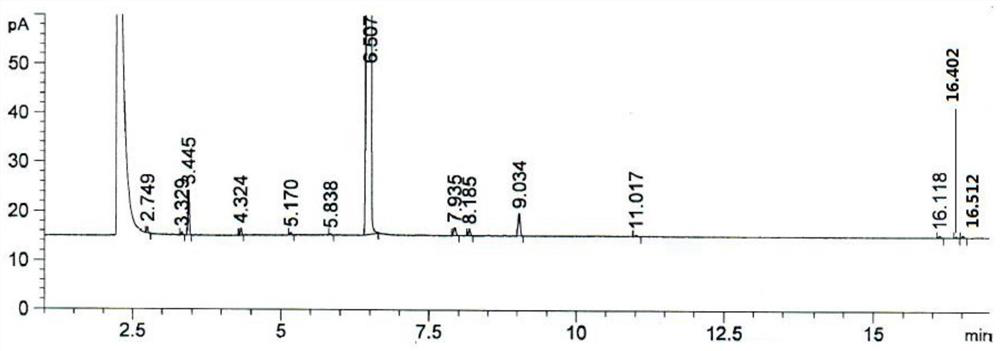
[0029] The invention provides a kind of preparation method of 4-bromo-1-methyl-2-(trifluoromethyl)benzene, comprising the following steps:
[0030] (1) Under an inert atmosphere, after mixing 2-methyl-5-aminobenzotrifluoride, aqueous hydrogen bromide, water and acetic acid, carry out a salt-forming reaction to obtain the bromine of 2-methyl-5-aminobenzotrifluoride Hydrogen salt;
[0031] (2) carry out diazotization reaction after the hydrogen bromide salt of described 2-methyl-5-aminobenzotrifluoride and sodium nitrite aqueous solution are mixed, obtain the diazonium of 2-methyl-5-aminobenzotrifluoride Salt;
[0032] (3) After mixing the diazonium salt of the 2-methyl-5-aminobenzotrifluoride, cuprous bromide and hydrogen bromide aqueous solution, carry out the Sandmeyer reaction to obtain 4-bromo-1-methyl- 2-(Trifluoromethyl)benzene.
[0033] In the present invention, all raw materials used in the preparation process can be commercially available products well known to thos...
Embodiment 1
[0058] Under nitrogen protection, add 50.1g48% hydrogen bromide aqueous solution, 33.4g water and 16.7g glacial acetic acid (pure acetic acid) at room temperature, stir, and dropwise add 16.7g2-methyl-5-aminotrifluorotoluene ( The isomer accounts for 0.037%, and the gas phase area is normalized), after the dropwise addition is completed, the temperature is raised to 93° C. and stirred for 30 minutes to obtain a hydrogen bromide salt solution.
[0059] Cool down to 3°C, a large amount of solids precipitated into a suspension, and 23.0g of 30% sodium nitrite aqueous solution was added dropwise to the suspension at 3°C. After the addition was complete, react at 3°C for 1.0h to obtain a diazonium salt solution.
[0060] The prepared diazonium salt solution was added dropwise to 6.8g of cuprous bromide and 94.8g of 48% hydrogen bromide aqueous solution prepared in advance at 66°C, a large amount of nitrogen gas was released, after the dropwise addition was completed, it was stirre...
Embodiment 2
[0065] Under nitrogen protection, add 2642.3g48% hydrogen bromide aqueous solution at room temperature, 1760.0g water and 880.0g acetic acid, stir, and dropwise add 880.0g2-methyl-5-amino-trifluorotoluene (isomer accounts for 0.037 %, normalized gas phase area), after the dropwise addition was completed, the temperature was raised to 95° C. and stirred for 30 min to obtain a hydrogen bromide salt solution.
[0066] Cool down to 0°C, and add 1213.3g of 30% sodium nitrite aqueous solution dropwise at 0°C, and react for 1.0h after the dropwise addition. The prepared diazonium salt was added dropwise to 360.4g of cuprous bromide and 4997.0g of 48% aqueous hydrogen bromide solution prepared in advance at 65°C, a large amount of nitrogen gas was released, and the dropwise addition was completed and stirred for 1 hour to obtain 4-bromo-1 -Methyl-2-(trifluoromethyl)benzene solution.
[0067] After the reaction, cool down to 20°C and stir for 1.0h, let stand, separate layers, add 1046...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com