Naphthalimide compound fluorescence probe for identifying Ag+ in HL-60 and its preparation method
A naphthalimide and fluorescent probe technology, applied in the field of metal ion fluorescent probes, can solve problems such as serious, easy to produce drug resistance, adverse reactions, etc., and achieve the effects of high response value, reliable detection method, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: This embodiment is a specific embodiment prepared according to the reaction scheme of the compound of formula (I).
[0034] (2-pyridyl)-4-(2-thiazolyl)-1.8-naphthalimide: add 1 mmol of 4-bromo-1,8-naphthalene anhydride to a 100 ml round bottom flask equipped with a reflux condenser , 1 mmol 2-aminopyridine, 2 mmol sodium hydroxide and 2 mmol copper chloride were dissolved in 20 ml of toluene, and the reaction temperature was set at 115 °C. With Schlenk Line TechnologyN 2Reflux under protection, TLC monitors the reaction, after 17 h, the reaction is complete. After standing overnight and standing at room temperature, a solid precipitated, filtered, and recrystallized with 40 ml of toluene to obtain compound (II) with a yield of 82%. Weigh 1 mmol of N-(2-pyridyl)-4-bromo-1,8-naphthimide, 1 mmol of 2-aminothiazole, 2 mmol of cesium carbonate and excess iodide of compound (II) Copper in 20 ml of DMF. With Schlenk Line TechnologyN 2 Reflux under protection...
Embodiment 2
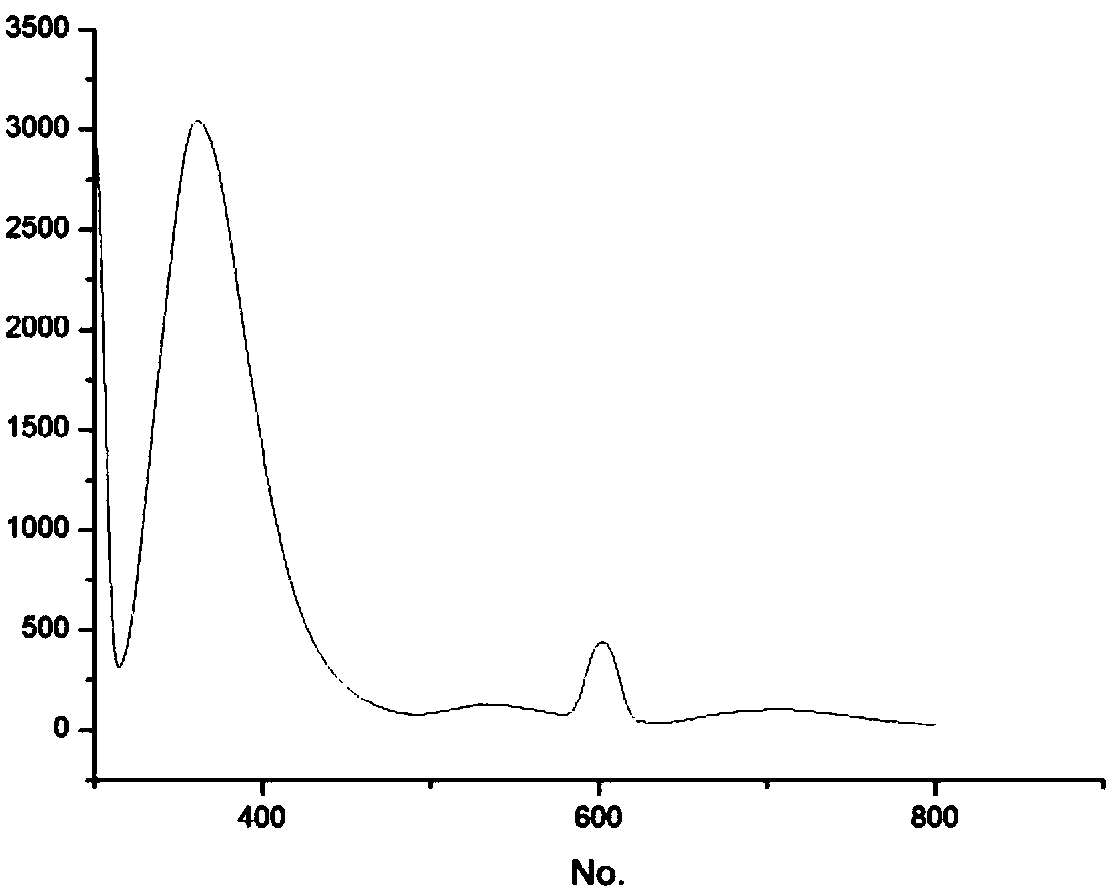
[0037] Embodiment 2: the ultraviolet absorption spectrogram of the N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthalimide that embodiment 1 makes (see figure 1 ) and fluorescence spectra (see Picture 1-1 ).
[0038] Accurately weigh N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthalimide, transfer it into a 10 mL volumetric flask, dissolve it with absolute ethanol, and keep to volume after the sample is completely dissolved , formulated to a concentration of 3.0×10 -5 mol•L -1 The solution was measured for its UV absorption spectrum and fluorescence excitation spectrum, respectively. Measurement results such as figure 1 , Picture 1-1 shown; figure 1 is the ultraviolet absorption spectrum of the silver ion fluorescent probe N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthalimide; Picture 1-1 It is the fluorescence spectrum of the silver ion fluorescent probe N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthalimide for the detection of aggregation-induced luminescent effect.
[0039] From figure 1...
Embodiment 3
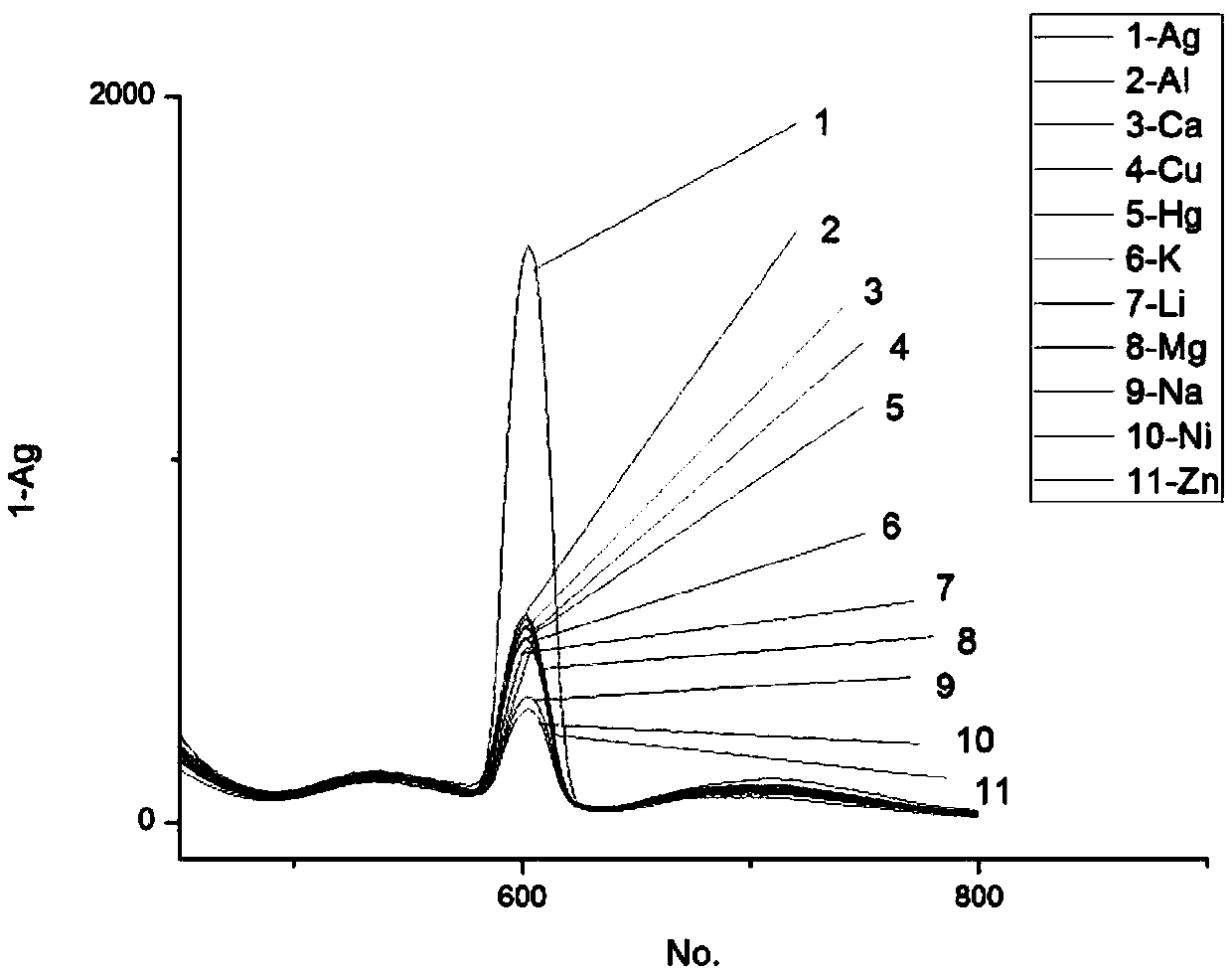
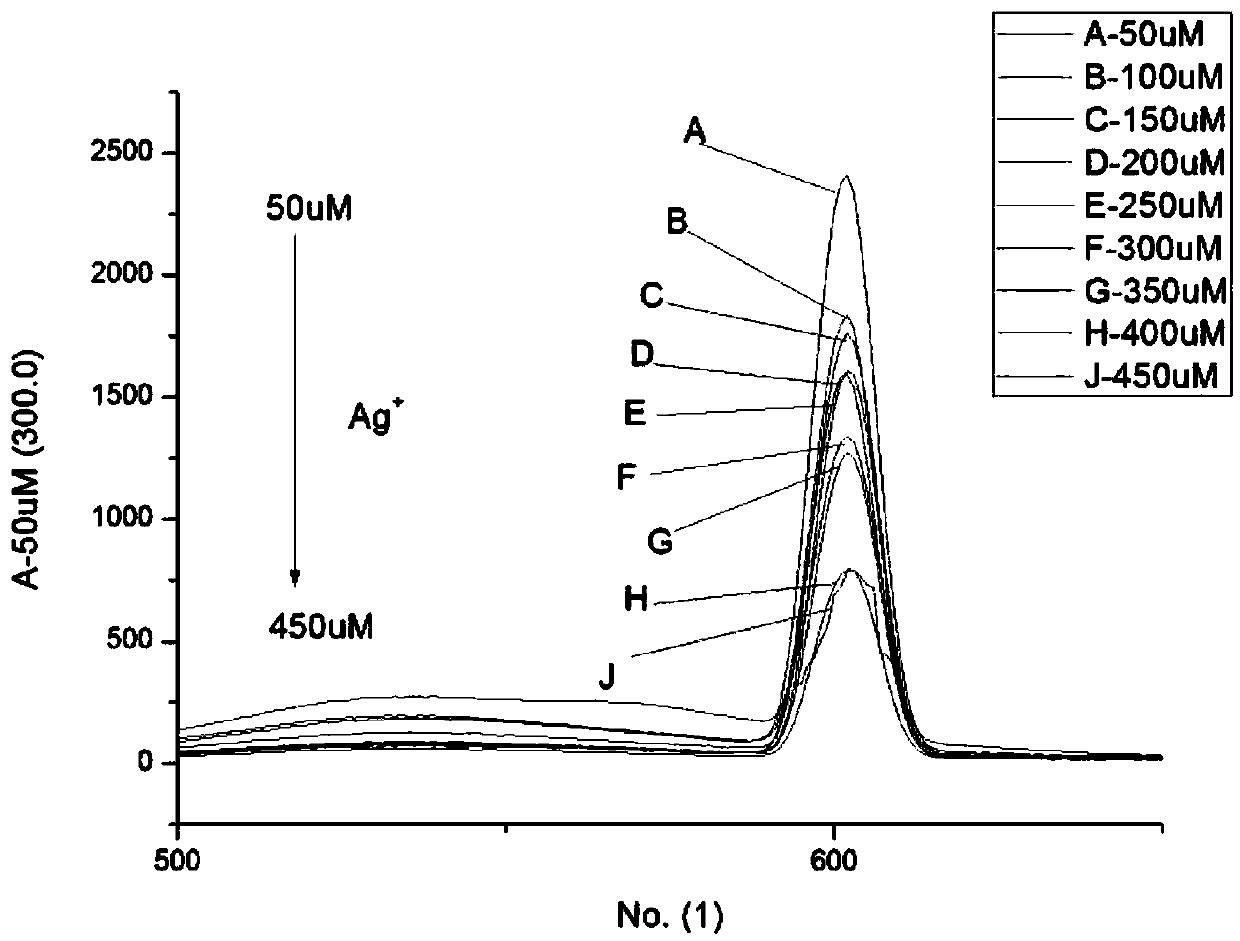
[0040] Example 3: The N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthoimide fluorescent probe prepared in Example 1 was measured at an excitation wavelength of 650 nm for Ag + selective.
[0041] Accurately weigh N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthalimide, dissolve it in chromatographically pure absolute ethanol, and configure it to a concentration of 1.0×10 -4 mol•L -1 In the ligand solution, 10 different metal ions (Zn 2+ , Hg + 、Cu 2+ , Mg 2+ , Li + 、Al 3+ 、Ni + , K + , Ca 2+ 、Ag + ), with a ligand concentration of 3.0×10 -5 mol•L -1 , metal ion concentration 1.0×10 -4 mol•L -1 The mixed solution was measured to measure the change of the fluorescence spectrum of the solution. Measurement results such as figure 2 shown.
[0042] From figure 2 It can be seen that Ag + The fluorescence intensity of N-(2-pyridyl)-4-(2-thiazolyl)-1.8-naphthoimide fluorescent probe is significantly stronger than that of other ions. + There is a high degree of selectivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com