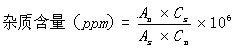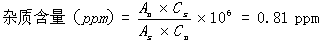LC-MS (liquid chromatography-mass spectrometry) detection method of 9-propenyladenine
A technology of acryl adenine and a detection method, which is applied in the field of medicine, can solve the problems that the sensitivity cannot meet the detection requirements, and is not effectively controlled, and achieves the effects of high sensitivity, simple method and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1 Selection of chromatographic conditions
[0027] 1.1 Selection of chromatographic column
[0028] Choose from three octadecylsilane-bonded silica columns. The retention behavior of different chromatographic columns is shown in Table 1:
[0029] Table 1 Retention behavior of 9-propenyl adenine in different chromatographic columns
[0030]
[0031] The results show that XBridge C18 (50mm×2.1mm , 3.5μm) interferes with the detection of genetic impurities in the starting material, and the other two columns can be used for the detection of genetic impurities, but in comparison, the YMC Pack ODS-AQ C18 (150mm×4.6mm, 3μm) chromatographic column has more appropriate retention time and higher sensitivity.
[0032] 1.2 Selection of column temperature
[0033] The commonly used column temperature was investigated, and the results are shown in Table 2:
[0034] Table 2 Effect of different column temperatures
[0035]
[0036] The results showed that the retention time...
Embodiment 2
[0059] 2 Establishment of methodology
[0060] 2.1 Specificity
[0061] Take the blank solvent, the control solution, the test solution and the mixed solution to inject samples respectively, and record the chromatograms. The results showed that the blank solvent, TDF and TDA did not interfere with the detection of 9-propenyladenine.
[0062] 2.2 Investigation of the stability of the sample solution
[0063] Take 1 part of the control solution, measure it at 0-8 hours respectively, record the peak area, and calculate RSD=2.17%, which shows that the control solution has good stability within 6 hours, and the intraday error is small. See Table 7:
[0064] Table 7 Stability of reference substance solution
[0065] .
[0066] 2.3 Limit of quantitation
[0067] Take an appropriate amount of 9-propenyl adenine reference substance, add methanol to make a solution with a certain concentration, and record the chromatogram. The limit of quantification is 5 ng / ml (S / N ≈ 10).
[0...
Embodiment 3
[0093] 3 Determination of 9-propenyl adenine in tenofovir disoproxil fumarate
[0094] (1) Chromatographic conditions
[0095] Chromatographic column: YMC Pack ODS-AQ C18, 150mm×4.6mm, 3μm;
[0096] Column temperature: 30 degrees;
[0097] m / z: 176;
[0098] Flow rate: 1ml / min
[0099] Mobile phase: 0.01mol / L ammonium acetate: acetonitrile=80:20;
[0100] (2) Preparation of the test sample and reference substance solution: take the test sample, accurately weigh it, add methanol to make a 2mg / ml solution, and use it as the test sample solution; take another 9-propenyl adenine reference substance, accurately weigh Weigh it, add methanol to make a 10ng / ml solution as a control solution;
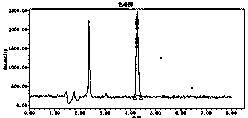
[0101] (3) Determination method: Accurately measure 10 μl of the reference substance solution and the test solution, inject the sample, record the chromatogram, and calculate the peak area according to the external standard method; if the test solution contains 9-propenyl adenine, it should...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com