External abamectin transdermal agent and preparation method thereof
A technology of abamectin and transdermal agent, which is applied in the field of external abamectin transdermal agent and its preparation, can solve the problems of inconvenient route of administration, large first-pass effect, etc., and achieve avoidance of hepatic first-pass effect and Gastrointestinal inactivation, high safety, and the effect of reducing individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
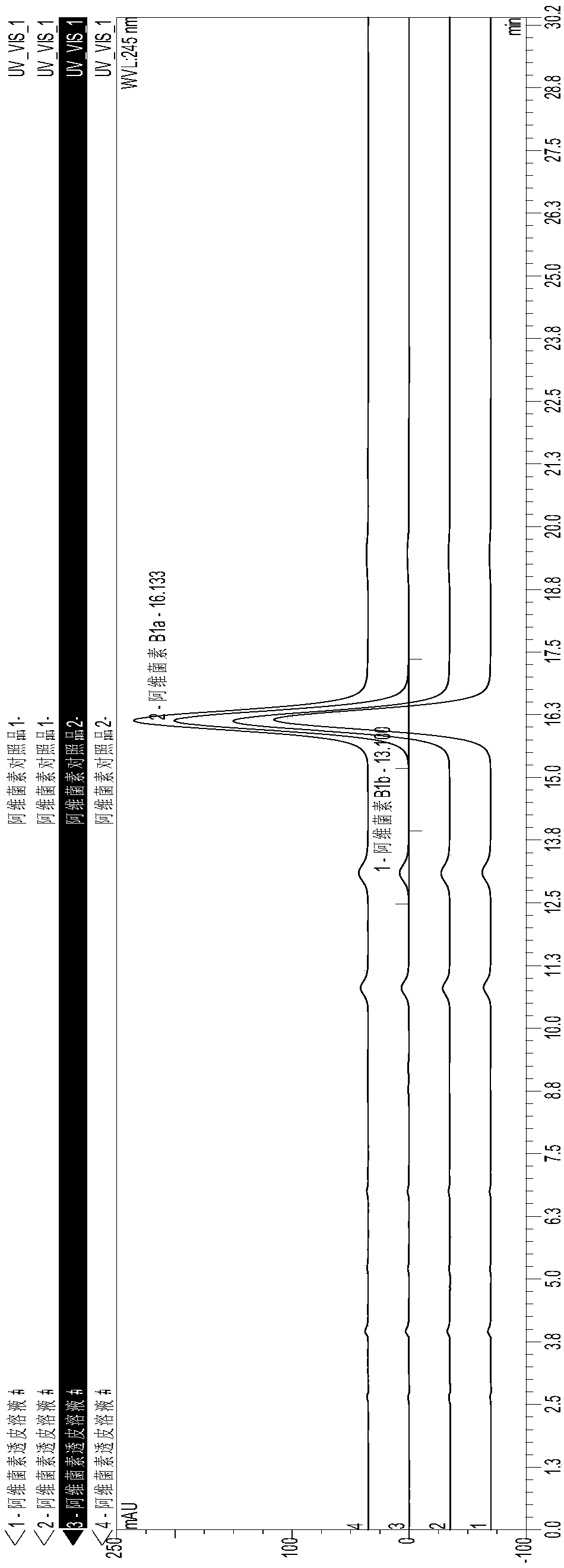
[0024] A kind of abamectin transdermal agent for external use and preparation method thereof, comprising the steps of: accurately weighing the abamectin B of formula quantity 1 , add 95% ethanol and stir to dissolve, then add azone, dimethyl sulfoxide, butylated hydroxyanisole (BHA) and stir evenly, and adjust the volume of soybean oil to 100 mL. The specific addition amount is shown in Table 1.
[0025] Table 1 A kind of Abamectin transdermal agent for external use prepares raw materials and its addition amount
[0026]
Embodiment 2
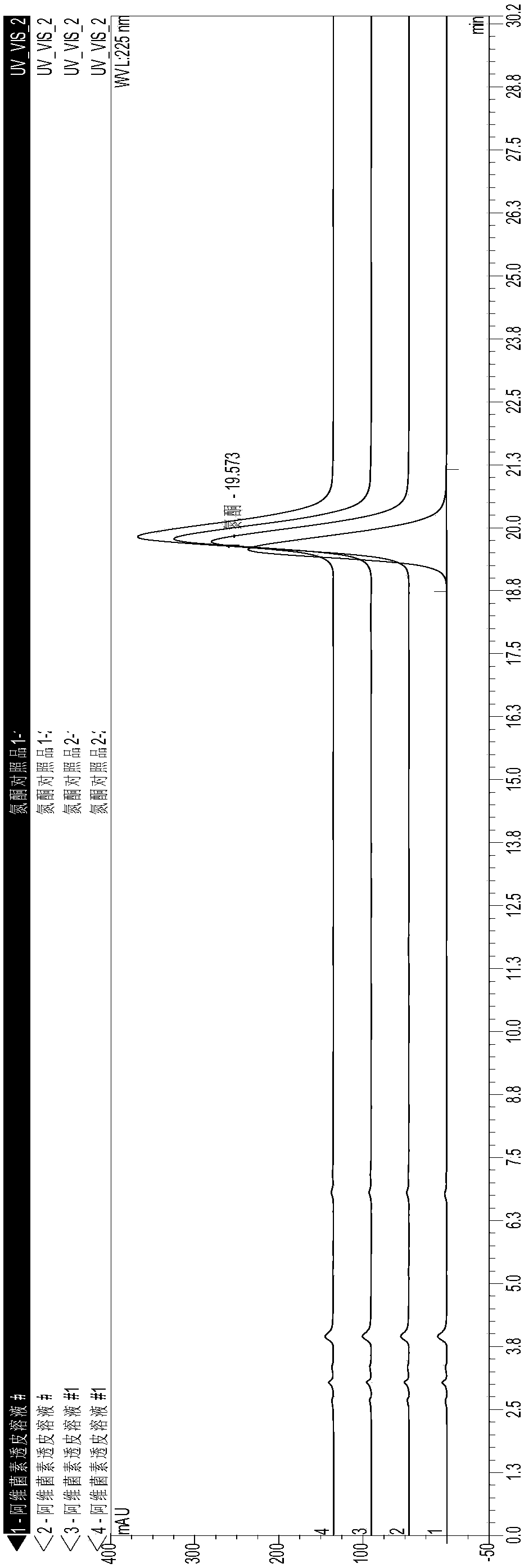
[0028] A kind of abamectin transdermal agent for external use and preparation method thereof, comprising the steps of: accurately weighing the abamectin B of formula quantity 1 , add isopropanol and stir to dissolve, then add azone, decylmethyl sulfoxide, butylated hydroxytoluene (BHT) and stir evenly, and dilute monoglyceride laurate to 100mL. The specific addition amount is shown in Table 2.
[0029] Table 2 A kind of external use Abamectin transdermal agent preparation raw material and its addition amount
[0030]
Embodiment 3
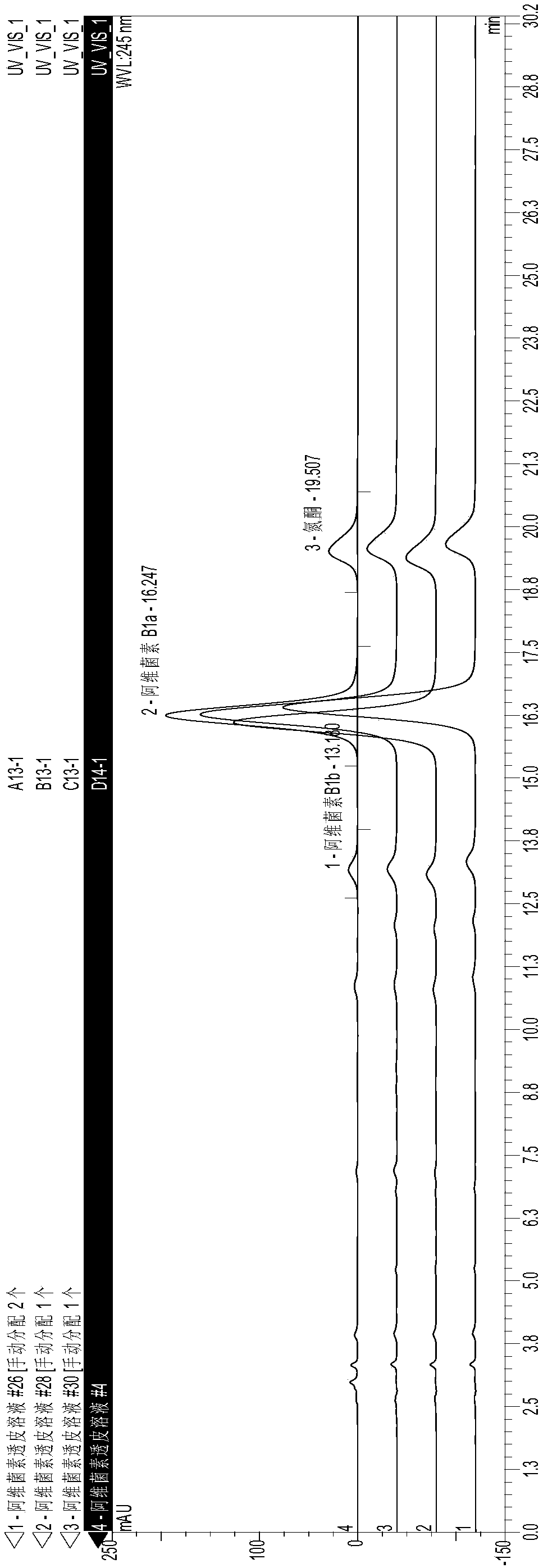
[0032] A kind of abamectin transdermal agent for external use and preparation method thereof, comprising the steps of: accurately weighing the abamectin B of formula quantity 1 , add ethyl oleate and stir to dissolve, then add azone, isopropyl myristate, and propyl gallate (PG) in sequence, stir evenly, and adjust the volume to 100 mL with propylene glycol. The specific addition amount is shown in Table 3.
[0033] Table 3 A kind of external use abamectin transdermal agent preparation raw material and its addition amount
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com