Piperaquine phosphate oral liquid and preparation method thereof
A kind of piperaquine phosphate oral liquid and piperaquine phosphate technology, applied in the field of medicine, can solve problems such as slow drug effect development time, and achieve the effects of low production process cost, low production cost, and improved quality and taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
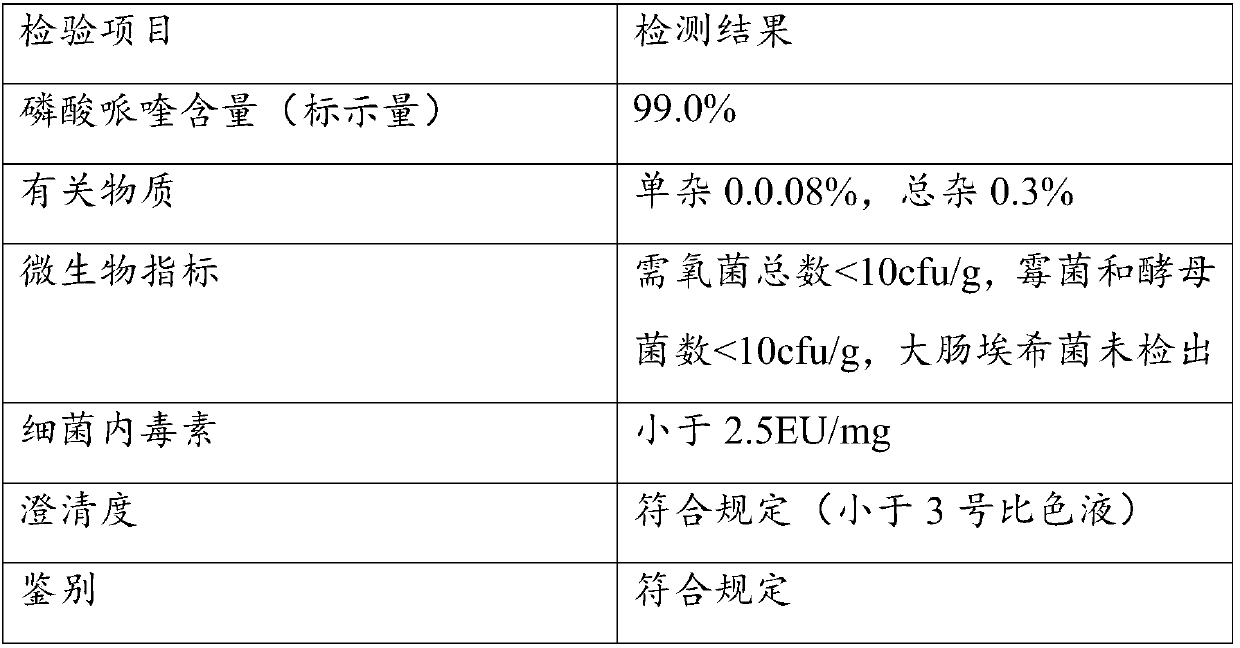
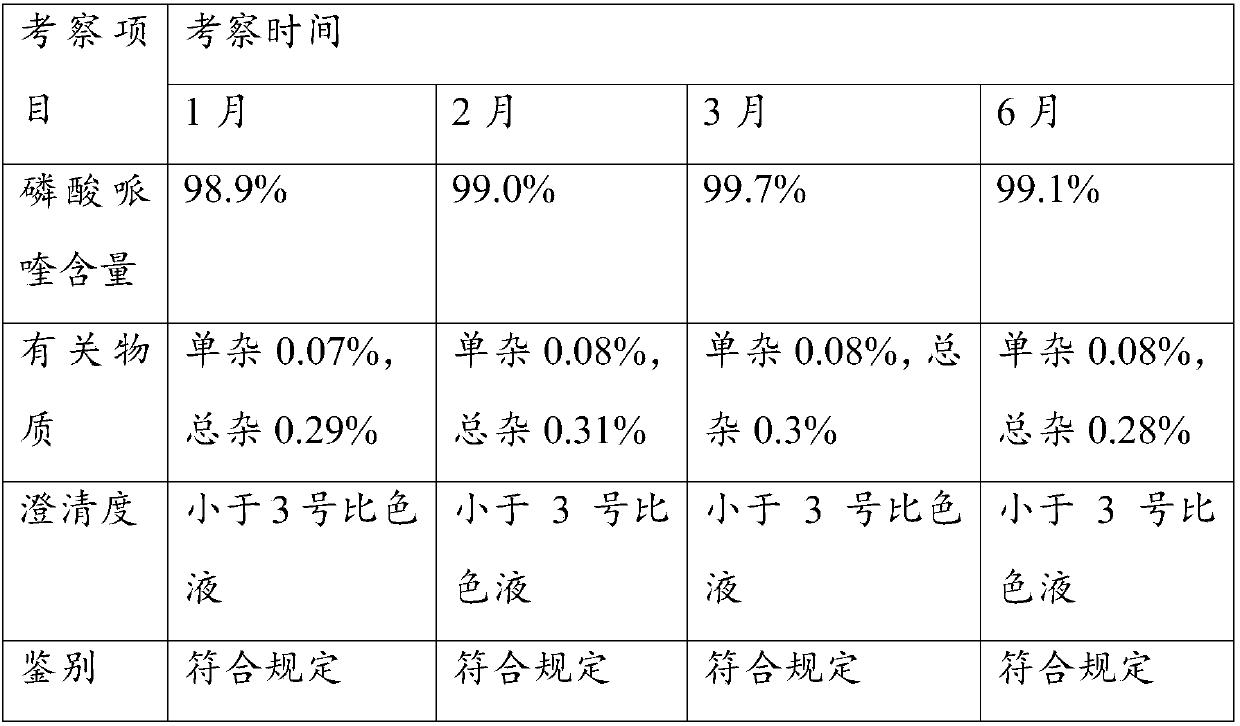
[0060] Weigh 10 g of piperaquine phosphate, add 50 mL of purified water, 10 g of ethanol, stir in a water bath at 50°C to 60°C to disperse evenly, add hydrochloric acid dropwise to control pH 2.5, dissolve piperaquine phosphate to obtain a transparent solution of piperaquine phosphate , add 0.5g sucralose and 0.1g butylated hydroxytoluene, keep at 50-60°C for 15 minutes, filter and cool to room temperature, add water to volume to 150mL to obtain a clear solution, which is divided into brown bottles, filtered, divided into Sterilize at 115°C for 20 min to prepare oral liquid.
Embodiment 2
[0062] Weigh 10 g of piperaquine phosphate, add 100 mL of purified water, 10 g of ethanol, stir in a water bath at 50°C to 60°C to make it evenly dispersed, add hydrochloric acid dropwise to control pH 2.5, dissolve piperaquine phosphate to obtain a transparent piperaquine phosphate solution , add 0.5g of sucralose and 0.1g of butylated hydroxytoluene, keep at 50-60°C for 15 minutes, filter and cool to room temperature, add water to make up to 200mL to obtain a clear solution, which is divided into brown bottles, filtered, divided into Sterilize at 115°C for 20 min to prepare oral liquid.
Embodiment 3
[0064] Weigh 10 g of piperaquine phosphate, add 100 mL of purified water, 10 g of ethanol, stir in a water bath at 50°C to 60°C to make it evenly dispersed, add hydrochloric acid dropwise to control pH 3.0, dissolve piperaquine phosphate to obtain a transparent piperaquine phosphate solution , add 0.5g of sucralose and 0.1g of butylated hydroxytoluene, keep at 50-60°C for 15 minutes, filter and cool to room temperature, add water to make up to 200mL to obtain a clear solution, which is divided into brown bottles, filtered, divided into Sterilize at 115°C for 20 min to prepare oral liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com