Method for synthesizing carfentrazone-ethyl
A technology of caroxafen-ethyl and phenyltriazolinone is applied in the field of chemistry, and can solve the problems of complicated substitution and elimination reaction operation steps, unfavorable industrial production, and difficulty in obtaining 2-isohydroxyethyl ethyl acrylate, and achieves the following advantages. Conducive to industrialized production, optimized synthesis method and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
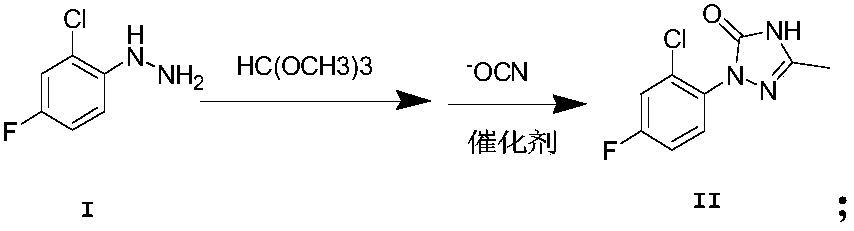
[0038] Example 1. Add 4-chloro-2-fluorophenylhydrazine and trimethyl orthoformate to methanol as a solvent, add triethylamine dropwise, heat to reflux, and detect the reaction by TLC until the reaction is complete. Put the above reaction solution in an ice-water bath On, then potassium cyanate was added, acetic acid was added dropwise, and then the catalyst (ureapropionic acid) was added and stirred until the reaction was complete.
[0039]
[0040] Raw material addition
[0041]
[0042] Effect of catalyst on reaction time and yield
[0043]
[0044] In the case of adding a catalyst, potassium cyanate and sodium cyanate are selected
[0045]
[0046] With the addition of a catalyst, phenylhydrazine and trimethyl orthoformate
[0047]
[0048] Product NMR data: 1 HNMR (400MHz, CDCl 3 , δppm): 2.48(s, 3H), 6.26(s, 1H), 7.02(t, J=58.0Hz, 1H), 7.35(d, J=9.6Hz, 1H), 7.97(d, J=7.8Hz , 1H).
Embodiment 2
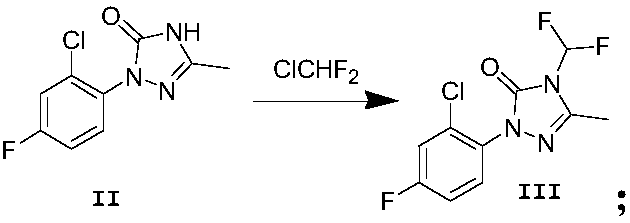
[0049] Example 2. The raw material phenyl-substituted triazolinone (6.9 g, 0.03 mol) was weighed in a 250 mL three-neck flask, and 50 mL of DMF was added to stir to dissolve it. The system temperature was heated to reflux, and potassium carbonate (6.90 g, 0.05 mol) was added. Continue to stir, the system turns into wine red, pass through chlorodifluoromethane gas, TLC traces the reaction after 20min, the obtained mixture is extracted twice with ethyl acetate, the organic layer is mixed, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a white solid compound III, 6.64g, yield 80%. 1 HNMR (400MHz, CDCl 3 , δppm): 2.48(s, 3H), 7.26(s, 1H), 7.02(t, J=58.0Hz, 1H), 7.35(d, J=9.6Hz, 1H), 7.97(d, J=7.8Hz , 1H).
Embodiment 3
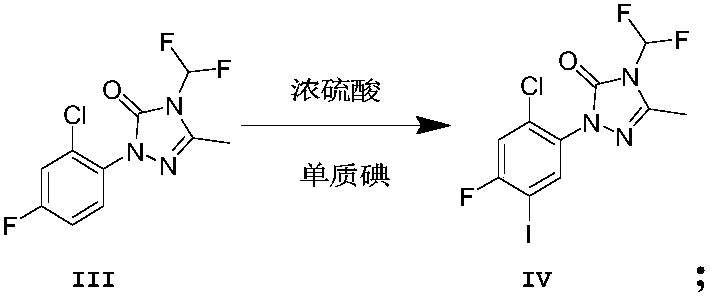
[0050] Example 3: Fluorinated triazolinone (13.9 g, 0.05 mol), concentrated sulfuric acid was added into an ice-salt bath, stirred until all solids were dissolved, and then iodine powder (12.7 g, 0.05 mol) was added. The mixture was then warmed to room temperature and stirred (reaction monitored by TLC). The reaction mixture was poured into 300 g of ice, and the obtained mixture was extracted twice with dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 18.33 g of compound IV as a white solid, with a yield of 91%. 1 HNMR (400MHz, CDCl 3 , δppm): 2.46 (s, 3H), 7.02 (t, J=58.0Hz, 1H), 7.35 (d, J=9.8Hz, 1H), 7.97 (d, J=7.6Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com