Signal peptide capable of effectively improving protein secretion efficiency and application of signal peptide
A protein secretion and signal peptide technology, applied in the field of genetic engineering, can solve problems such as difficulty in secretion and expression of heterologous proteins, and achieve the effects of increased secretion, secretion and expression, and improved enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Screening of Signal Peptides Efficiently Secreted and Expressed by β-galactosidase
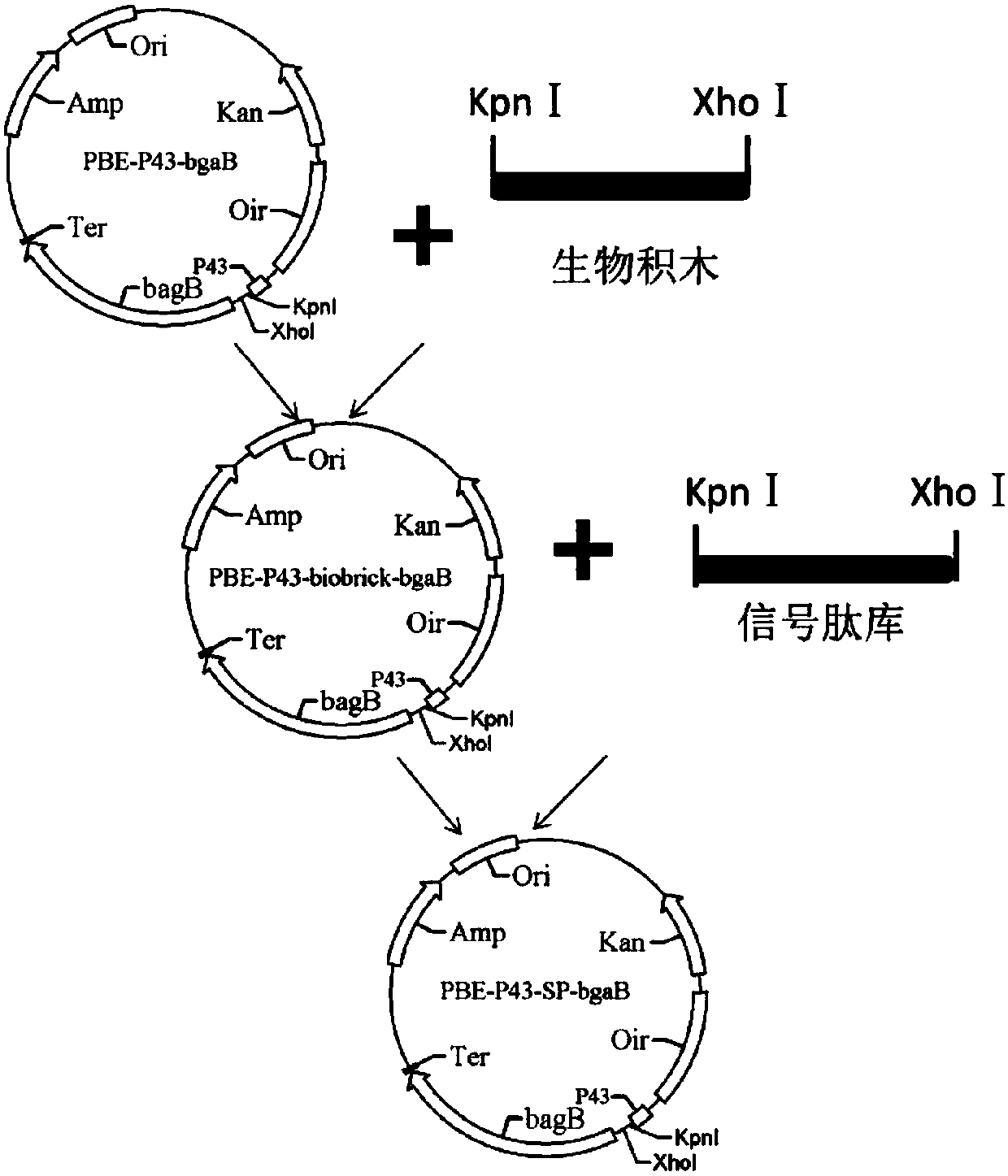
[0029] With reference to the method of (Christian Degering et.Al, 2010), by constructing the expression vector that various signal peptides combine with β-galactosidase coding gene (bgaB) from Bacillus subtilis homologous protein signal peptide library, combine A high-throughput screening method to obtain signal peptides that improve the secretion effect of β-galactosidase to varying degrees from clones, specifically including the following steps:
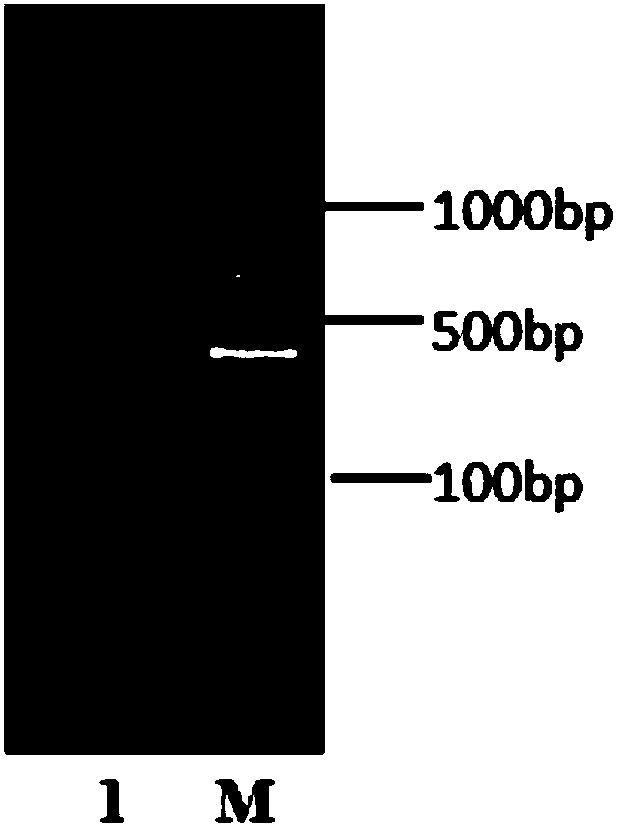
[0030] (1) Construction of pBEp43-biobrick-bgaB vector: The PCR fragment of biobrick (biological building block) obtained by annealing and extending two artificially synthesized fragments of SEQ ID NO.3 and SEQ ID NO.4 has restriction sites KpnI and XhoI, and used Restriction endonuclease digested and purified 100bp PCR product (such as figure 1 ) was inserted into the plasmid pBE-P43-bgaB (according to the patent literature: Pan ...
Embodiment 2
[0035] The detection of embodiment 2 signal peptide expression levels
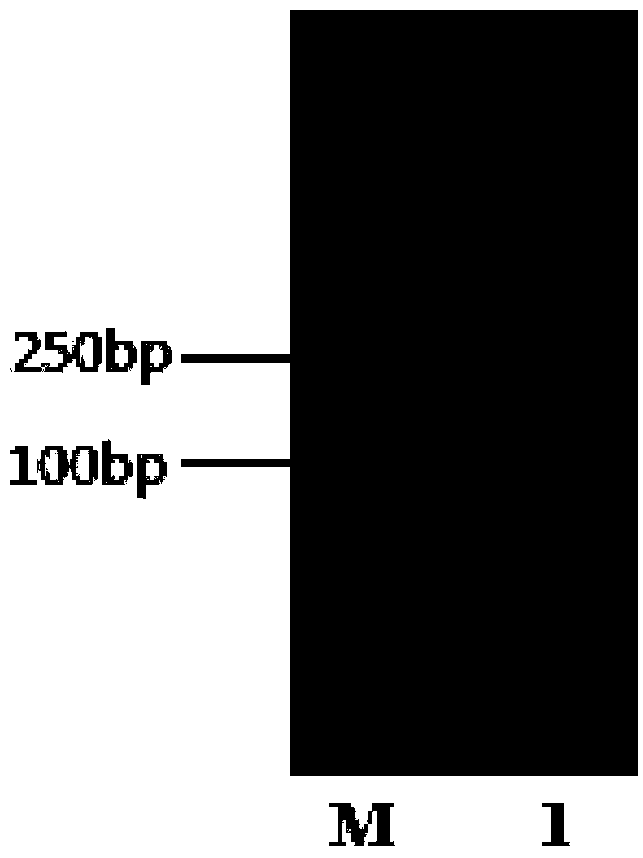
[0036] (1) Construction of MTG (transglutaminase) extracellular expression plasmid: the plasmid pBEp43-proMTG (according to the patent literature: Pan Li et al. A recombinant Bacillus subtilis and its method for producing transglutaminase. CN201210052578 .2 [P]. 2012. Construction) as an expression plasmid, using the pBEp43-SPyoqM-bgaB plasmid obtained in the above-mentioned Example 1 as a template, primers F-SPyoqM (5'-GAGAGGAATGTCGACATGAA ATTAAGAAAAGTATTGACTG-3'), R-SPyoqM (5 '-CGTTGTCCATCTCGAGAGCGAATGCAGGAGAAGCAGAAAC-3') to amplify about 100bp SPyoqM fragment (see image 3 ). The signal peptide (SPyoqM fragment) was connected with the linearized plasmid (pBEp43-proMTG) by the In-fusion method (see the HiFi DNA Assembly Master Mix of NEBuilder for the specific operation method), and the MTG expression extracellular plasmid pBEp43-SPyoqM-proMTG was constructed (see Figure 4 ). First transform into Esc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com